Europe Software as a Medical Devices Market to Grow at a CAGR of 21.6% to reach US$ 24,898.07 Million from 2020 to 2027
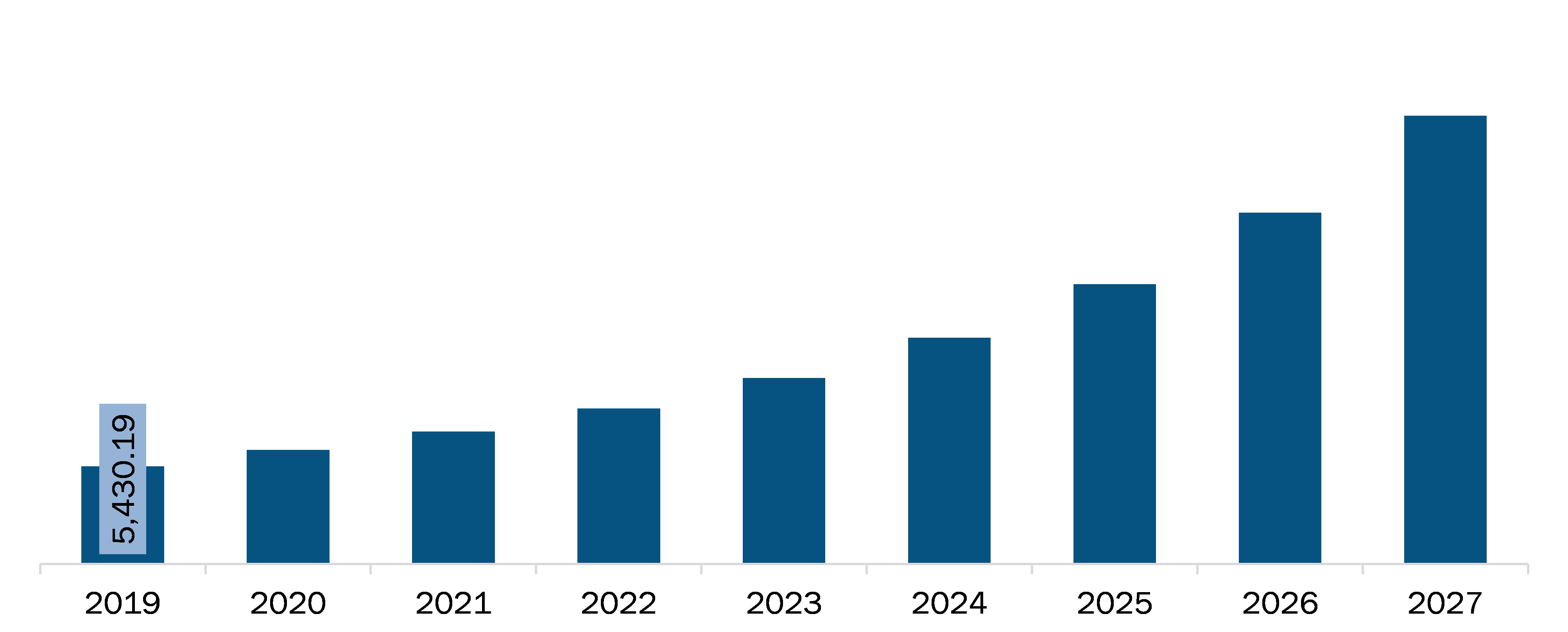
The Europe software as a medical devices market is expected to reach US$ 24,898.07 million by 2027 from US$ 5,430.19 million in 2019. The market is estimated to grow at a CAGR of 21.6% from 2020 to 2027.
The growth of the Europe software as a medical devices market is attributed to the growing adoption of wearable devices, increasing investments in connected devices & healthcare IoT, and rising emphasis on utilization of intelligent platforms to improve healthcare outcomes. However, the increased risk of data breach limits the growth of market in this region. The COVID-19 has shown a significant impact on the Europe software as a medical devices market. Factors such as increasing number of coronavirus cases and growing number of fatalities due to the virus are leading to complete shutdown of businesses across the region, leading to hampering the growth of the market.The Europe software as a medical devices market is expected to witness significant growth during the forecast period due to increasing adoption of IoT in healthcare. Applications for IoT in healthcare majorly includes continuous monitoring of physiological activities such as blood pressure, body temperature, pulse rate, respiratory rates and others. Furthermore, IoT is also used for continuous data collection of certain parameters of patients suffering with chronic illness to provide remote assistance. The monitoring is evolved nowadays with the installation of specific apps in the patient’s smartphone or wearable device that acts as both the sensor and tracker converting it into a medical device to perform the task saving cost of monitoring. The above-mentioned factors are thus likely expected to account for the growth of software as medical device market in the years to come. In March 2015, Aceton introduced X-Mind Prime as an addition to their previously existing 2D and 3D panoramic device line. The compact wall-mounted system is likely to provide dental practitioners with various new operational efficiencies. The rising number of technologically advanced dental X-rays is expected to be supportive in propelling the growth of the market.
Germany is among the major countries in the software as a medical devices market in this region. The governments in the country pay for a higher amount of national healthcare spending as compared to developing countries. The government has taken active participation in the development and regularization of the digitalization in healthcare in Germany. For instance, in 2019, the German government has initiated a law ‘Digital Care Act’ to digitalize the healthcare system. This act is based on the ‘e-Health Act’ in 2016. The main objective of this new law to develop the ‘telematics infrastructure’ for healthcare services providers. Such constructive activities are likely to drive the software as a medical devices market in the country.
Europe Software as a medical devices market Revenue and Forecast to 2027 (US$ Million)
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
- Request discounts available for Start-Ups & Universities
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
- Request discounts available for Start-Ups & Universities
Europe Software as a medical devices market Segmentation
By Device Type
- Smartphone/Tablets
- Wearable Devices
- PCs/Laptop
By Application
- Screening and Diagnosis
- Monitoring and Alerting
- Disease Management
By Deployment
- Cloud
- On-Premise
By Country
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe
Company Profiles
- Velentium LLC
- Tietronix Software Inc.
- S3 Connected Health
- Zühlke Group
- Science Group

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Device Type, Application, Deployment Type, and Country

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
UK, Germany, France, Italy, Russia
1. Introduction
1.1 Scope of the Study
1.2 The Insight Partners Research Report Guidance
1.3 Market Segmentation
1.3.1 Europe Software as a Medical Device Market – By Device Type
1.3.2 Europe Software as a Medical Device Market – By Application
1.3.3 Europe Software as a Medical Device Market – By Deployment Type
1.3.4 Europe Software as a Medical Device Market – By Region
2. Software as a Medical Device Market – Key Takeaways
3. Research Methodology
3.1 Coverage
3.2 Secondary Research
3.3 Primary Research
4. Software As A Medical Device– Market Landscape
4.1 Overview
4.2 PEST Analysis
4.2.1 Europe
4.3 Expert Opinion
5. Europe Software as a Medical Device Market – Industry Dynamics
5.1 Key Drivers
5.1.1 Increasing Adoption of IoT in Healthcare
5.1.2 Advantages of Software as a Medical Device (SaMD)
5.2 Key Market Restraints
5.2.1 Threat of Data Breach
5.3 Key Market Opportunities
5.3.1 Connected Healthcare in Europe
5.4 Future Trends
5.4.1 Increasing Number of Wearable Devices
5.5 Impact Analysis
6. Software as a Medical Device Market – Regional Analysis
6.1 Europe Software as a Medical Device Market Revenue Forecast and Analysis
7. Software as a Medical Device Market Analysis – By Product
7.1 Overview
7.2 Software as a Medical Device Market Revenue Share, by Device Type, 2019 and 2027 (%)
7.2.1 Europe Software as a Medical Device Market , by Device Types– Revenue and Forecast to 2027 (USD Million)
7.3 PCs/Laptop
7.3.1 Overview
7.3.2 PCs/Laptops: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
7.4 Smartphones/Tablets
7.4.1 Overview
7.4.2 Smartphones/Tablets: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
7.5 Wearable Devices
7.5.1 Overview
7.5.2 Wearable Devices: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
8. Software as a medical device market – By Application
8.1 Overview
8.2 Software as a Medical Device Market, by Application, 2019 and 2027 (%)
8.2.1 Europe Software as a Medical Device Market, by Application – Revenue and Forecast to 2027 (USD Million)
8.3 Screening and Diagnosis
8.3.1 Overview
8.3.2 Screening and Diagnosis: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
8.4 Monitoring and Alerting
8.4.1 Overview
8.4.2 Monitoring and Altering: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
8.5 Disease Management
8.5.1 Overview
8.5.2 Disease Management: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
9. Software as a Medical Device Market Analysis – By Deployment Type
9.1 Overview
9.2 Software as a Medical Device Market Revenue Share, by Deployment Type, 2019 and 2027 (%)
9.2.1 Europe Software as a Medical Device Market , by Deployment Type– Revenue and Forecast to 2027 (USD Million)
9.3 Cloud
9.3.1 Overview
9.3.2 Cloud: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
9.4 On-Premise
9.4.1 Overview
9.4.2 On-Premise: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
10. Software as a Medical Device Market – Regional Analysis
10.1 Europe: Software as a Medical Device Market
10.1.1 Overview
10.1.2 Europe: Software as s Medical Device (SaMD) Market - Revenue and Forecast to 2027 (USD Million)
10.1.3 Europe: Software as s Medical Device (SaMD) Market, by Device Type, 2018–2027 (USD Million)
10.1.4 Europe: Software as s Medical Device (SaMD) Market, by Application, 2018–2027 (USD Million)
10.1.5 Europe: Software as s Medical Device (SaMD) Market, by Deployment Type, 2018–2027 (USD Million)
10.1.6 Europe: Software as s Medical Device (SaMD) Market, by Country, 2018 & 2027 (%)
10.1.7 Germany: Software as s Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
10.1.7.1 Germany: Software as s Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
10.1.7.2 Germany: Software as s Medical Device (SaMD) Market Device Type, 2018–2027 (USD Million)
10.1.7.3 Germany: Software as s Medical Device (SaMD) Market, by Application, 2018–2027 (USD Million)
10.1.7.4 Germany: Software as s Medical Device (SaMD) Market, by Deployment Type, 2018–2027 (USD Million)
10.1.7.5 Germany: Software as s Medical Device (SaMD) Market, by Device Type, 2018–2027 (USD Million)
10.1.8 UK: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
10.1.8.1 UK: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
10.1.8.2 UK: Software as s Medical Device (SaMD) Market, by Device Type, 2018–2027 (USD Million)
10.1.8.3 UK: Software as a Medical Device (SaMD) Market Device Type, 2018–2027 (USD Million)
10.1.8.4 United Kingdom: Software as s Medical Device (SaMD) Market, by Application, 2018–2027 (USD Million)
10.1.8.5 United Kingdom: Software as s Medical Device (SaMD) Market, by Deployment Type, 2018–2027 (USD Million)
10.1.9 France: Software as s Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
10.1.9.1 France: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
10.1.9.2 France: Software as a Medical Device (SaMD) Market Device Type, 2018–2027 (USD Million)
10.1.9.3 France: Software as s Medical Device (SaMD) Market, by Application, 2018–2027 (USD Million)
10.1.9.4 France: Software as s Medical Device (SaMD) Market, by Deployment Type, 2018–2027 (USD Million)
10.1.10 Spain: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
10.1.10.1 Spain: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
10.1.10.2 Spain: Software as s Medical Device (SaMD) Market Device Type, 2018–2027 (USD Million)
10.1.10.3 Spain: Software as s Medical Device (SaMD) Market, by Application, 2018–2027 (USD Million)
10.1.10.4 Spain: Software as s Medical Device (SaMD) Market, by Deployment Type, 2018–2027 (USD Million)
10.1.11 Italy: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
10.1.11.1 Italy: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
10.1.11.2 Italy: Software as a Medical Device (SaMD) Market Device Type, 2018–2027 (USD Million)
10.1.11.3 Italy: Software as s Medical Device (SaMD) Market, by Application, 2018–2027 (USD Million)
10.1.11.4 Italy: Software as s Medical Device (SaMD) Market, by Deployment Type, 2018–2027 (USD Million)
11. Impact of COVID-19 Pandemic on Europe Software as a Medical Device Market
11.1 Europe: Impact assessment of COVID-19 Pandemic
12. Company Profiles
12.1 Velentium LLC
12.1.1 Key Facts
12.1.2 Business Description
12.1.3 Products and Services
12.1.4 Financial Overview
12.1.5 SWOT Analysis
12.1.6 Key Developments
12.2 Tietronix Software Inc.
12.2.1 Key Facts
12.2.2 Business Description
12.2.3 Products and Services
12.2.4 Financial Overview
12.2.5 SWOT Analysis
12.2.6 Key Developments
12.3 S3 Connected Health
12.3.1 Key Facts
12.3.2 Business Description
12.3.3 Products and Services
12.3.4 Financial Overview
12.3.5 SWOT Analysis
12.3.6 Key Developments
12.4 Zühlke Group
12.4.1 Key Facts
12.4.2 Business Description
12.4.3 Products and Services
12.4.4 Financial Overview
12.4.5 SWOT Analysis
12.4.6 Key Developments
12.5 Science Group
12.5.1 Key Facts
12.5.2 Business Description
12.5.3 Products and Services
12.5.4 Financial Overview
12.5.5 SWOT Analysis
12.5.6 Key Developments
13. Appendix
13.1 About The Insight Partners
13.2 Glossary of Terms
LIST OF TABLES
Table 1. Europe Software as a Medical Device Market , by Device Types – Revenue and Forecast to 2027 (USD Million)
Table 2. Europe Software as a Medical Device Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 3. Europe Software as a Medical Device Market , by Deployment Type – Revenue and Forecast to 2027 (USD Million)
Table 4. Table: 2
Table 5. Europe Software as s Medical Device (SaMD Market, by Device Type – Revenue and Forecast to 2027 (USD Million)
Table 6. Europe Software as s Medical Device (SaMD) Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 7. Europe Software as s Medical Device (SaMD) Market, by Sample Type – Revenue and Forecast to 2027 (USD Million)
Table 8. Germany Software as s Medical Device (SaMD) Market Device Type – Revenue and Forecast to 2027 (USD Million)
Table 9. Germany Software as s Medical Device (SaMD) Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 10. Germany Software as s Medical Device (SaMD) Market, by Deployment Type – Revenue and Forecast to 2027 (USD Million)
Table 11. Germany Software as s Medical Device (SaMD) Market, by Device Type– Revenue and Forecast to 2027 (USD Million)
Table 12. UK Software as s Medical Device (SaMD) Market, by Device Type– Revenue and Forecast to 2027 (USD Million)
Table 13. UK Software as s Medical Device (SaMD) Market Device Type – Revenue and Forecast to 2027 (USD Million)
Table 14. UK Software as s Medical Device (SaMD) Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 15. UK Software as s Medical Device (SaMD) Market, by Deployment Type – Revenue and Forecast to 2027 (USD Million)
Table 16. France Software as s Medical Device (SaMD) Market Device Type – Revenue and Forecast to 2027 (USD Million)
Table 17. France Software as s Medical Device (SaMD) Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 18. France Software as s Medical Device (SaMD) Market, by Deployment Type – Revenue and Forecast to 2027 (USD Million)
Table 19. Spain Software as s Medical Device (SaMD) Market Device Type – Revenue and Forecast to 2027 (USD Million)
Table 20. Spain Software as s Medical Device (SaMD) Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 21. Spain Software as s Medical Device (SaMD) Market, by Deployment Type – Revenue and Forecast to 2027 (USD Million)
Table 22. Italy Software as s Medical Device (SaMD) Market Device Type – Revenue and Forecast to 2027 (USD Million)
Table 23. Italy Software as s Medical Device (SaMD) Market, by Application – Revenue and Forecast to 2027 (USD Million)
Table 24. Italy Software as s Medical Device (SaMD) Market, by Deployment Type – Revenue and Forecast to 2027 (USD Million)
Table 25. Glossary of Terms, Software as a medical device market
LIST OF FIGURES
Figure 1. Software as a Medical Device Market Segmentation
Figure 2. Software as a medical device market Segmentation – By Region
Figure 3. Europe Software as a Medical Device Market Overview
Figure 4. The Wearable Devices Segment Held Largest Share of Device Type Segment In Software as a Medical Device Market
Figure 5. Germany is Expected to Show Remarkable Growth During the Forecast Period
Figure 6. Europe PEST Analysis
Figure 7. Software as a Medical Device Market Impact Analysis of Drivers and Restraints
Figure 8. Europe Software as a Medical Device Market – Revenue Forecast and Analysis – 2019- 2027
Figure 9. Software as a Medical Device Market Revenue Share, by Device Type, 2019 and 2027 (%)
Figure 10. PCs/Laptops: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
Figure 11. Smartphones/Tablets: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
Figure 12. Wearable Devices: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
Figure 13. Software as a Medical Device Market, by Application, 2019 and 2027 (%)
Figure 14. Screening and Diagnosis: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
Figure 15. Monitoring and Alerting: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
Figure 16. Disease Management: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
Figure 17. Software as a Medical Device Market Revenue Share, by Deployment Type, 2019 and 2027 (%)
Figure 18. Cloud: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
Figure 19. On-Premise: Software as a Medical Device Market – Revenue and Forecast to 2027 (US$ Million)
Figure 20. Europe: Software as a Medical Device Market, by Key Country – Revenue (2019) (USD Million)
Figure 21. Europe Software as s Medical Device (SaMD) Market Revenue and Forecast to 2027 (USD Million)
Figure 22. Europe: Software as s Medical Device (SaMD) Market, by Country, 2018 & 2027 (%)
Figure 23. Germany: Software as s Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
Figure 24. UK: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
Figure 25. France: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
Figure 26. Spain: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
Figure 27. Italy: Software as a Medical Device (SaMD) Market – Revenue and Forecast to 2027 (USD Million)
Figure 28. Impact of COVID-19 Pandemic in Europe Country Markets
- Velentium LLC
- Tietronix Software Inc.
- S3 Connected Health
- Zühlke Group
- Science Group
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
 Get Free Sample For
Get Free Sample For