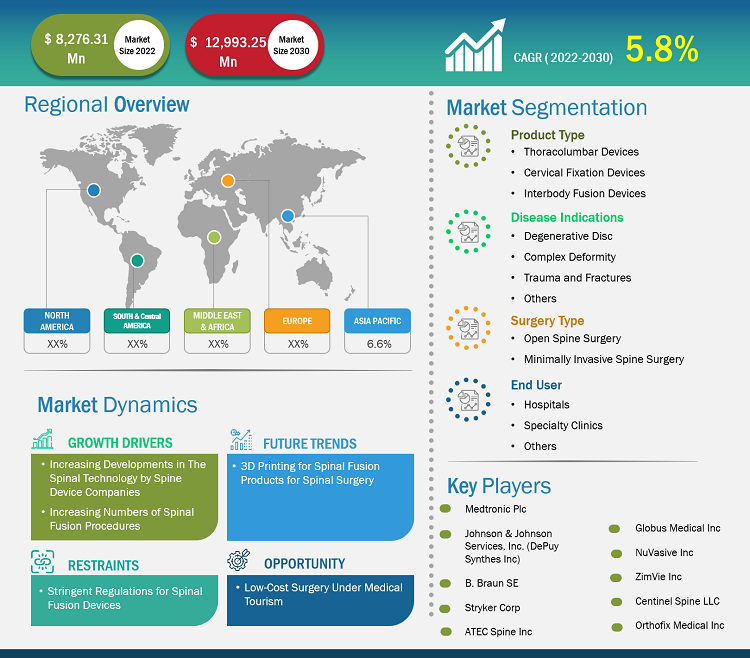
[Research Report] The spinal fusion devices market size is expected to grow from US$ 8,276.31 million in 2022 and to reach a value of US$ 12,993.25 million by 2030, it is anticipated to record a CAGR of 5.8% from 2022 to 2030.
Market Insights and Analyst View:
The spinal fusion devices market size is growing rapidly due to the increase in number of spinal fusion devices cases due to increase in cases of gallstones. Also, increase in cases of diabetes fuel the growth of the market.
In addition, strategic initiatives by companies for the market development are fueling the growth of the market. In September 2022, GE Healthcare announced US FDA 510(k) clearance of its breakthrough AIR Recon DL for 3D and PROPELLER imaging sequences. The advantages of AIR Recon DL are extended by these new features to almost all Magnetic Resonance Imaging (MRI) clinical procedures, covering all anatomies and enabling better image quality, shorter scan times, and enhanced patient experience. Thus, a significant rise in revolutionary technologies to address customer needs is likely to bring new trends in the market during the forecast period.
Opportunities and Challenges:
Spinal fusion surgeries are much costlier; many patients slide back from the decision to undergo spinal surgeries. Also, in many cases, the cost of spinal implants is not covered under health insurance plans, limiting the number of surgeries. For instance, a country like the US is expensive; according to the Healthcare Bluebook data published in February 2022, the top 5 cities are listed with the cost of lumbar spinal fusion: Denver: US$ 86,182, San Jose, California: US$ 78,809 San Francisco: US$ 78,809, Indianapolis: US$ 77,269, and Seattle: US$ 74,499. At the same time, the least cost for lumbar spinal fusion is ~ US$ 48,000 in cities such as Memphis, Tenn, and San Antonio.
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Spinal Fusion Devices Market: Strategic Insights
Market Size Value in US$ 8,276.31 million in 2022 Market Size Value by US$ 12,993.25 million by 2030 Growth rate CAGR of 5.8% from 2022 to 2030 Forecast Period 2022-2030 Base Year 2022

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Spinal Fusion Devices Market: Strategic Insights

| Market Size Value in | US$ 8,276.31 million in 2022 |
| Market Size Value by | US$ 12,993.25 million by 2030 |
| Growth rate | CAGR of 5.8% from 2022 to 2030 |
| Forecast Period | 2022-2030 |
| Base Year | 2022 |

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
On the other hand, medical tourism has enabled to offer spinal fusion surgeries at cheaper costs. Countries in developing regions have significantly developed medical tourism and have advanced technologies that provide world-class medical services at cheaper rates. Lyfboat Technologies Pvt. data published on July 2023 stated that countries such as India, Thailand, Turkey, the UAE, and Egypt are considered best for offering scoliosis surgery between US$ 8,000 and US$ 16,000. The data published by Lyfboat Technologies Pvt also reveals the cost of spinal fusion surgery in Egypt is US$ 10,897, and in the UAE, it is ~ US$ 8,200. Similarly, in Turkey, the cost of spinal fusion surgery is US$ 15,000 and in India is nearly between US$ 8000 and US$ 12,000. Also, the cost of thoracoplasty in India is between US$ 2,500 and US$ 4,000.
Offering critical surgeries at cheaper costs increases patient flow to these countries for their medical treatments. Also, the availability of advanced medical techniques and increasing government funding to increase medical tourism are leading to the increasing demand for advanced surgical implants. Further, the advancing healthcare infrastructure in developing countries will likely continue the demand for advanced medical devices, enhancing growth opportunities for the spinal fusion devices market.
Regulatory requirements for spinal fusion devices are different from the other implantable devices. The regulations for spinal fusion devices are regularly updated to maintain the quality of products to offer enhanced quality of life to patients. In March 2023, the Food and Drug Administration (FDA) updated medical devices, orthopedic devices, classification of spinal spheres for use in intervertebral fusion procedures (final rule) final regulatory impact analysis. According to the FDA’s analysis, spinal devices are classified as Class III. The devices will require separate filing for pre-market approval applications. Moreover, the FDA found that general and special controls are insufficient to assure the devices' effectiveness and safety. Therefore, it is expected that companies must provide complete product descriptions and analyses for product efficiency and safety to avoid confusion concerning the product’s material and specifications for sizes and intentions of clinical condition.
Similarly, the regulations in developing countries are updating regulatory requirements for spinal fusion devices. In November 2021, the Australian government's Department of Health and Aged Care Therapeutic Goods Administration (TGA) revised the regulatory framework for spinal implantable medical devices. TGA aims to support sponsors and manufacturers with new regulatory requirements to understand and comply with the updated requirements. TGA has proposed four major requirements for the manufacturers, which include the need for specific information in the ARTG entry about Class IIb spinal fusion devices; TGA’s mandatory audit assessment for device inclusion applications, including assessment of clinical evidence; conformity assessment documents that demonstrate appropriate procedures for device classification, and manufacturer’s exhaustive assessment for the quality management systems and technical documentation related to each device. Thus, the strictness of the regulations for manufacturers will lead to more economic investments, and the risk of recalls may lead to losses. Therefore, the stringent regulations for spinal fusion devices are among the factors hindering the market growth.
Report Segmentation and Scope:
The spinal fusion devices market share is divided on the basis of product type, surgery type, disease indication, and end user. The spinal fusion devices market, by product type, is segmented into thoracolumbar devices, cervical fixation devices, and interbody fusion devices. The spinal fusion devices market, by surgery type, is bifurcated into open spine surgery and minimally invasive spine surgery. The spinal fusion devices market, by disease indications, is segmented into degenerative disc, trauma and fractures, complex deformity, and others. The spinal fusion devices market, by end user, is segment into hospitals, specialty clinics, others. Based on geography, the market is divided into North America (the US, Canada, and Mexico), Europe (the UK, Germany, France, Italy, Spain, and the Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific), Middle East & Africa (the UAE, Saudi Arabia, South Africa, and the Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Segmental Analysis:
Based on surgery type, the Spinal Fusion Devices Market is bifurcated into open spine surgery and minimally invasive spine surgery. In 2022, the open spine surgery segment held a larger share of the market by surgery. The minimally invasive spine surgery segment is estimated to grow at a higher CAGR during 2022–2030 due to increasing adoption of minimally invasive spine surgery approach. Open surgeries are considered standard surgeries as they offer complete exposure to the anatomy. Open spine surgery is recommended to treat conditions such as scoliosis, severe degeneration of the discs, spine instability, or a combination of these problems. Open surgery is widely preferred if the condition is severe and complex; it provides a larger exposure to anatomy, offering ease for the procedure and greater visibility of surrounding structures. However, there are several risks associated with open spine surgery type. The risk includes excessive blood loss and longer recovery time.
In many cases, open spine surgery type may reverse the symptoms of spinal disorders. Complications may also lead to infection, poor wound healing, blood clots, and damage to surrounding veins or nerves.
Minimally invasive spine surgery type (MISS) has gained notable attention over traditional open spine surgery type. MISS requires smaller incisions; therefore, convincing patients to undergo MISS is easier. Other advantages such as avoiding damage to the surrounding muscles, less bleeding, less pain, faster recovery, and shorter hospital stays have increased the adoption of MISS. At present, MISS is considered a common procedure for spinal fusion. The higher adoption of MISS includes treating spinal problems in older and critically sick people by limiting the risks and changing reimbursement patterns and patient preferences. In addition, transformations in MISS have introduced new intraoperative imaging techniques by combining powerful software. The combination of imaging techniques and software allows real-time navigation for surgeons to supplement their understanding of the spine's 3-dimensional (3D) anatomy. Thus, the transformation in MISS is leading to the segment's fastest growth, eventually driving market growth. According to an article titled “Minimally Invasive Spine Surgery Type: An Overview,” published in July 2022, ~75% of 1.2 million spine procedures in the US are performed annually by MISS techniques. Similarly, an article, “Spinal Endoscopy: Evidence, Techniques, Global Trends, and Future Projections,” published in January 2022, stated that 96.7% of Asian surgeons perform MISS. Thus, considering the increasing number of MISS procedures, the market is anticipated to grow notably in the coming years.
The spinal fusion devices market, by product type, is segmented into thoracolumbar devices, cervical fixation devices, and interbody fusion devices. In 2022, the thoracolumbar devices segment held the largest share of the market by product type. The interbody fusion devices segment is estimated to grow at a significant CAGR during 2022–2030 due to growing developments for the interbody fusion devices. The spinal fusion devices market, by disease indication, is categorized into degenerative disc, trauma and fractures, complex deformity, and others. In 2022, the degenerative disc segment held the largest share of the market by disease indications and is estimated to grow at a significant CAGR during 2022–2030 due to growing geriatric population that are more prone to degenerative diseases. The spinal fusion devices market, by end user, is segment into hospitals, specialty clinics, others. In 2022, the hospitals segment held the largest share of the market and is expected to grow at the fastest rate during the coming years.
Regional Analysis:
Based on geography, the spinal fusion devices market is divided into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America. North America is the largest contributor to the growth of the market, and Asia Pacific is the fastest-growing region. The spinal fusion devices market in North America is segmented into the US, Canada, and Mexico. In 2022, the US held the largest market share in this region and is expected to continue its dominance during the forecast period. DePuy Synthes, Stryker, Aurora Spine, and Alevio Spine are among the major players operating in the spinal fusion devices market in the US. Product developments and launches driven by these players favor the market growth. Technologically advanced spinal fusion devices approved by the Food and Drug Administration (FDA) are widely adopted in the US. Following is the list of spinal fusion devices recently approved by the FDA:
- In May 2023, CTL Amedica received FDA 510(k) clearance for the commercialization of the NITRO Interbody Fusion Cage System, which is exclusively made by the fusion of biomaterial silicon nitride. Silicon nitride material is compatible with all imaging modalities; it exhibits unique bacteriostatic properties and provides artifact-free imaging.
- In January 2023, Alevio Spine received 510 (K) clearance of additional indications for the SI-Cure SI Joint Fusion System. The expanded indication includes sacroiliac fusion for skeletally mature patients undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion.
- In June 2022, the US FDA granted 510K clearance for Aurora Spine’s DEXA SOLO-L anterior lumbar interbody fusion device (ALIF). Based on DEXA Technology Platform, a 3D printed standalone device was designed for anterior and lateral lumbar interbody fusion (ALIF & LLIF) procedures.
Age-related wear-and-tear triggers the prevalence of lower back pain (LBP) among the geriatric population in the US, in turn, fuels the demand for spinal fusion devices. According to National Health Services in 2022, lifetime incidence of LBP in the US is reported to be 60–90%, with annual incidence of 5%. The source also states that 14.3% of new patients visit physicians each year because of LBP, and ~13 million people visit physician due to chronic LBP.
Spinal Fusion Devices Market Report Scope
Industry Developments and Future Opportunities:
Various initiatives by key players operating in the Spinal Fusion Devices Market are listed below:
- In February 2023, Globus Medical Inc, a prominent musculoskeletal solutions provider, and NUVASIVE, a pioneer in spine technology innovation, agreed to merge in an all-stock transaction. The transaction brings together two well-known musculoskeletal technology companies with a common vision of innovation in the relentless pursuit of unmet clinical requirements to improve patient care.
- In November 2022, Centinel Spine LLC announced the first implantation of its prodisc C SK Cervical Total Disc Replacement (TDR) product. The prodisc C SK system is the second of the three new products to be released.
- In March 2022, DePuy Synthes Inc acquired CUPTIMIZE Hip-Spine Analysis, providing surgeons with an easy-to-use tool for better understanding and addressing the impact of aberrant mobility between the spine and pelvis in some patients requiring total hip arthroplasty (THA). CUPTIMIZE Hip-Spine Analysis improves the surgical planning capabilities of VELYS Hip Navigation, one of DePuy Synthes' VELYS Digital Surgery platforms of connected technologies.
Competitive Landscape and Key Companies:
A few prominent players operating in the spinal fusion devices market are Medtronic PLC, Johnson & Johnson Services Inc, B.Braun SE, Stryker Corp, ATEC Spine Inc, Globus Medical Inc, NuVasive Inc, ZimVie Inc, Centinel Spine LLC, and Orthofix Medical Inc. These companies focus on new product launches and geographic expansions to meet the growing consumer demand worldwide and increase their product range in specialty portfolios. Their global presence allows them to serve a large set of customers, subsequently allowing them to expand their market share.

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product Type, Surgery Type, Disease Indications, End User, and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Companies operating in the market are Medtronic PLC, Johnson & Johnson Services Inc, B.Braun SE, Stryker Corp, ATEC Spine Inc, Globus Medical Inc, NuVasive Inc, ZimVie Inc, Centinel Spine LLC, and Orthofix Medical Inc.
The spinal fusion devices market, by product type, is bifurcated into thoracolumbar devices, cervical fixation devices, and interbody fusion devices. In 2022, the thoracolumbar devices segment held the largest share of the market by product type. And interbody fusion devices segment is estimated to grow at a significant CAGR during 2022-2030.
The factors driving the market include the increasing developments in the spinal technology by spine device companies and increasing numbers of spinal fusion procedures.
Global spinal fusion devices market is segmented by region into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America. North America is likely to continue its dominance in the spinal fusion devices market during 2022–2030. The US holds the largest share of the market in North America and is expected to continue this trend during the forecast period.
The spinal fusion devices market, by surgery type, is bifurcated into open spine surgery and minimally invasive spine surgery. In 2022, the open spine surgery segment held the largest share of the market by surgery. And minimally invasive spine surgery segment is estimated to grow at a significant CAGR during 2022-2030.
Spinal fusion is surgery to connect two or more bones in any part of the spine. Connecting them prevents movement between them. Preventing movement helps to prevent pain. During spinal fusion, a surgeon places bone or a bonelike material in the space between two spinal bones. Metal plates, screws or rods might hold the bones together. They then can fuse and heal as one bone.
1. Introduction
1.1 The Insight Partners Research Report Guidance
1.2 Market Segmentation
2. Executive Summary
2.1 Key Insights
3. Research Methodology
3.1 Coverage
3.2 Secondary Research
3.3 Primary Research
4. Spinal Fusion Devices Market Landscape
4.1 Overview
4.2 PEST Analysis
4.2.1 Global PEST Analysis
4.3 Ecosystem Analysis
4.3.1 List of Vendors in the Value Chain
5. Spinal Fusion Devices Market - Key Industry Dynamics
5.1 Market Drivers
5.1.1 Increasing Developments in Spinal Technology by Spine Device Companies
5.1.2 Surging Number of Spinal Fusion Procedures
5.2 Market Restraints
5.2.1 Stringent Regulations for Spinal Fusion Devices
5.3 Market Opportunities
5.3.1 Low-Cost Surgery Under Medical Tourism
5.4 Market Trends
5.4.1 3D Printing for Spinal Fusion Products for Spinal Surgery
5.5 Impact Analysis:
6. Spinal Fusion Devices Market - Global Market Analysis
6.1 Spinal Fusion Devices Market Revenue (US$ Mn), 2022 – 2030
7. Global Spinal Fusion Devices Market – Revenue and Forecast to 2030 – by Product Type
7.1 Overview
7.2 Spinal Fusion Devices Market Revenue Share, by Product Type, 2022 & 2030 (%)
7.3 Thoracolumbar Devices
7.3.1 Overview
7.3.2 Thoracolumbar Devices: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
7.3.3 Anterior Lumbar Plates
7.3.3.1 Overview
7.3.3.2 Anterior Lumbar Plates: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
7.3.4 Pedicle Screw and Rods
7.3.4.1 Overview
7.3.4.2 Pedicle Screw and Rods: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
7.3.5 Others
7.3.5.1 Overview
7.3.5.2 Others: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
7.4 Cervical Fixation Devices
7.4.1 Overview
7.4.2 Cervical Fixation Devices: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
7.4.3 Anterior Cervical Plates
7.4.3.1 Overview
7.4.3.2 Anterior Cervical Plates: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
7.4.4 Hook Fixation Systems
7.4.4.1 Overview
7.4.4.2 Hook Fixation Systems: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
7.4.5 Others
7.4.5.1 Overview
7.4.5.2 Others: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
7.5 Interbody Fusion Devices
7.5.1 Overview
7.5.2 Interbody Fusion Devices: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
8. Global Spinal Fusion Devices Market – Revenue and Forecast to 2030 – by Surgery Type
8.1 Overview
8.2 Spinal Fusion Devices Market Revenue Share, by Surgery Type, 2022 & 2030 (%)
8.3 Open Spine Surgery
8.3.1 Overview
8.3.2 Open Spine Surgery: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
8.4 Minimally Invasive Spine Surgery
8.4.1 Overview
8.4.2 Minimally Invasive Spine Surgery: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
9. Global Spinal Fusion Devices Market – Revenue and Forecast to 2030 – by Disease Indications
9.1 Overview
9.2 Spinal Fusion Devices Market Revenue Share, by Disease Indications 2022 & 2030 (%)
9.3 Degenerative Disc
9.3.1 Overview
9.3.2 Degenerative Disc: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
9.4 Trauma and Fractures
9.4.1 Overview
9.4.2 Trauma and Fractures: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
9.5 Complex Deformity
9.5.1 Overview
9.5.2 Complex Deformity: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
9.6 Others
9.6.1 Overview
9.6.2 Others: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
10. Global Spinal Fusion Devices Market – Revenue and Forecast to 2030 – by End User
10.1 Overview
10.2 Spinal Fusion Devices Market Revenue Share, by End User 2022 & 2030 (%)
10.3 Hospitals
10.3.1 Overview
10.3.2 Hospitals: Spinal Fusion Devices Market– Revenue and Forecast to 2030 (US$ Million)
10.4 Specialty Clinics
10.4.1 Overview
10.4.2 Specialty Clinics: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
10.5 Others
10.5.1 Overview
10.5.2 Others: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
11. Spinal Fusion Devices Market - Geographical Analysis
11.1 North America Spinal Fusion Devices Market, Revenue and Forecast To 2030
11.1.1 Overview
11.1.2 North America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.1.3 North America Spinal Fusion Devices Market, by Product Type
11.1.3.1 North America Spinal Fusion Devices Market, by Thoracolumbar Devices
11.1.3.2 North America Spinal Fusion Devices Market, by Cervical Fixation Devices
11.1.4 North America Spinal Fusion Devices Market, by Disease Indication
11.1.5 North America Spinal Fusion Devices Market, by Surgery Type
11.1.6 North America Spinal Fusion Devices Market, by End User
11.1.7 North America Spinal Fusion Devices Market, by Country
11.1.7.1 US
11.1.7.1.1 Overview
11.1.7.1.2 US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.1.7.1.3 US Spinal Fusion Devices Market, by Product Type
11.1.7.1.3.1 US Spinal Fusion Devices Market, by Thoracolumbar Devices
11.1.7.1.3.2 US Spinal Fusion Devices Market, by Cervical Fixation Devices
11.1.7.1.4 US Spinal Fusion Devices Market, by Disease Indication
11.1.7.1.5 US Spinal Fusion Devices Market, by Surgery Type
11.1.7.1.6 US Spinal Fusion Devices Market, by End User
11.1.7.2 Canada
11.1.7.2.1 Overview
11.1.7.2.2 Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.1.7.2.3 Canada Spinal Fusion Devices Market, by Product Type
11.1.7.2.3.1 Canada Spinal Fusion Devices Market, by Thoracolumbar Devices
11.1.7.2.3.2 Canada Spinal Fusion Devices Market, by Cervical Fixation Devices
11.1.7.2.4 Canada Spinal Fusion Devices Market, by Disease Indication
11.1.7.2.5 Canada Spinal Fusion Devices Market, by Surgery Type
11.1.7.2.6 Canada Spinal Fusion Devices Market, by End User
11.1.7.3 Mexico
11.1.7.3.1 Overview
11.1.7.3.2 Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.1.7.3.3 Mexico Spinal Fusion Devices Market, by Product Type
11.1.7.3.3.1 Mexico Spinal Fusion Devices Market, by Thoracolumbar Devices
11.1.7.3.3.2 Mexico Spinal Fusion Devices Market, by Cervical Fixation Devices
11.1.7.3.4 Mexico Spinal Fusion Devices Market, by Disease Indication
11.1.7.3.5 Mexico Spinal Fusion Devices Market, by Surgery Type
11.1.7.3.6 Mexico Spinal Fusion Devices Market, by End User
11.2 Europe Spinal Fusion Devices Market, Revenue and Forecast To 2030
11.2.1 Overview
11.2.2 Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.2.3 Europe Spinal Fusion Devices Market, by Product Type
11.2.3.1 Europe Spinal Fusion Devices Market, by Thoracolumbar Devices
11.2.3.2 Europe Spinal Fusion Devices Market, by Cervical Fixation Devices
11.2.4 Europe Spinal Fusion Devices Market, by Disease Indication
11.2.5 Europe Spinal Fusion Devices Market, by Surgery Type
11.2.6 Europe Spinal Fusion Devices Market, by End User
11.2.7 Europe Spinal Fusion Devices Market, by Country
11.2.7.1 UK
11.2.7.1.1 Overview
11.2.7.1.2 UK Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.2.7.1.3 UK Spinal Fusion Devices Market, by Product Type
11.2.7.1.3.1 UK Spinal Fusion Devices Market, by Thoracolumbar Devices
11.2.7.1.3.2 UK Spinal Fusion Devices Market, by Cervical Fixation Devices
11.2.7.1.4 UK Spinal Fusion Devices Market, by Disease Indication
11.2.7.1.5 UK Spinal Fusion Devices Market, by Surgery Type
11.2.7.1.6 UK Spinal Fusion Devices Market, by End User
11.2.7.2 Germany
11.2.7.2.1 Overview
11.2.7.2.2 Germany Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.2.7.2.3 Germany Spinal Fusion Devices Market, by Product Type
11.2.7.2.3.1 Germany Spinal Fusion Devices Market, by Thoracolumbar Devices
11.2.7.2.3.2 Germany Spinal Fusion Devices Market, by Cervical Fixation Devices
11.2.7.2.4 Germany Spinal Fusion Devices Market, by Disease Indication
11.2.7.2.5 Germany Spinal Fusion Devices Market, by Surgery Type
11.2.7.2.6 Germany Spinal Fusion Devices Market, by End User
11.2.7.3 France
11.2.7.3.1 Overview
11.2.7.3.2 France Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.2.7.3.3 France Spinal Fusion Devices Market, by Product Type
11.2.7.3.3.1 France Spinal Fusion Devices Market, by Thoracolumbar Devices
11.2.7.3.3.2 France Spinal Fusion Devices Market, by Cervical Fixation Devices
11.2.7.3.4 France Spinal Fusion Devices Market, by Disease Indication
11.2.7.3.5 France Spinal Fusion Devices Market, by Surgery Type
11.2.7.3.6 France Spinal Fusion Devices Market, by End User
11.2.7.4 Italy
11.2.7.4.1 Overview
11.2.7.4.2 Italy Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.2.7.4.3 Italy Spinal Fusion Devices Market, by Product Type
11.2.7.4.3.1 Italy Spinal Fusion Devices Market, by Thoracolumbar Devices
11.2.7.4.3.2 Italy Spinal Fusion Devices Market, by Cervical Fixation Devices
11.2.7.4.4 Italy Spinal Fusion Devices Market, by Disease Indication
11.2.7.4.5 Italy Spinal Fusion Devices Market, by Surgery Type
11.2.7.4.6 Italy Spinal Fusion Devices Market, by End User
11.2.7.5 Spain
11.2.7.5.1 Overview
11.2.7.5.2 Spain Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.2.7.5.3 Spain Spinal Fusion Devices Market, by Product Type
11.2.7.5.3.1 Spain Spinal Fusion Devices Market, by Thoracolumbar Devices
11.2.7.5.3.2 Spain Spinal Fusion Devices Market, by Cervical Fixation Devices
11.2.7.5.4 Spain Spinal Fusion Devices Market, by Disease Indication
11.2.7.5.5 Spain Spinal Fusion Devices Market, by Surgery Type
11.2.7.5.6 Spain Spinal Fusion Devices Market, by End User
11.2.7.6 Rest of Europe
11.2.7.6.1 Overview
11.2.7.6.2 Rest of Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.2.7.6.3 Rest of Europe Spinal Fusion Devices Market, by Product Type
11.2.7.6.3.1 Rest of Europe Spinal Fusion Devices Market, by Thoracolumbar Devices
11.2.7.6.3.2 Rest of Europe Spinal Fusion Devices Market, by Cervical Fixation Devices
11.2.7.6.4 Rest of Europe Spinal Fusion Devices Market, by Disease Indication
11.2.7.6.5 Rest of Europe Spinal Fusion Devices Market, by Surgery Type
11.2.7.6.6 Rest of Europe Spinal Fusion Devices Market, by End User
11.3 Asia Pacific Spinal Fusion Devices Market, Revenue and Forecast To 2030
11.3.1 Overview
11.3.2 Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.3.3 Asia Pacific Spinal Fusion Devices Market, by Product Type
11.3.3.1 Asia Pacific Spinal Fusion Devices Market, by Thoracolumbar Devices
11.3.3.2 Asia Pacific Spinal Fusion Devices Market, by Cervical Fixation Devices
11.3.4 Asia Pacific Spinal Fusion Devices Market, by Disease Indication
11.3.5 Asia Pacific Spinal Fusion Devices Market, by Surgery Type
11.3.6 Asia Pacific Spinal Fusion Devices Market, by End User
11.3.7 Asia Pacific Spinal Fusion Devices Market, by Country
11.3.7.1 China
11.3.7.1.1 Overview
11.3.7.1.2 China Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.3.7.1.3 China Spinal Fusion Devices Market, by Product Type
11.3.7.1.3.1 China Spinal Fusion Devices Market, by Thoracolumbar Devices
11.3.7.1.3.2 China Spinal Fusion Devices Market, by Cervical Fixation Devices
11.3.7.1.4 China Spinal Fusion Devices Market, by Disease Indication
11.3.7.1.5 China Spinal Fusion Devices Market, by Surgery Type
11.3.7.1.6 China Spinal Fusion Devices Market, by End User
11.3.7.2 Japan
11.3.7.2.1 Overview
11.3.7.2.2 Japan Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.3.7.2.3 Japan Spinal Fusion Devices Market, by Product Type
11.3.7.2.3.1 Japan Spinal Fusion Devices Market, by Thoracolumbar Devices
11.3.7.2.3.2 Japan Spinal Fusion Devices Market, by Cervical Fixation Devices
11.3.7.2.4 Japan Spinal Fusion Devices Market, by Disease Indication
11.3.7.2.5 Japan Spinal Fusion Devices Market, by Surgery Type
11.3.7.2.6 Japan Spinal Fusion Devices Market, by End User
11.3.7.3 India
11.3.7.3.1 Overview
11.3.7.3.2 India Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.3.7.3.3 India Spinal Fusion Devices Market, by Product Type
11.3.7.3.3.1 India Spinal Fusion Devices Market, by Thoracolumbar Devices
11.3.7.3.3.2 India Spinal Fusion Devices Market, by Cervical Fixation Devices
11.3.7.3.4 India Spinal Fusion Devices Market, by Disease Indication
11.3.7.3.5 India Spinal Fusion Devices Market, by Surgery Type
11.3.7.3.6 India Spinal Fusion Devices Market, by End User
11.3.7.4 Australia
11.3.7.4.1 Overview
11.3.7.4.2 Australia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.3.7.4.3 Australia Spinal Fusion Devices Market, by Product Type
11.3.7.4.3.1 Australia Spinal Fusion Devices Market, by Thoracolumbar Devices
11.3.7.4.3.2 Australia Spinal Fusion Devices Market, by Cervical Fixation Devices
11.3.7.4.4 Australia Spinal Fusion Devices Market, by Disease Indication
11.3.7.4.5 Australia Spinal Fusion Devices Market, by Surgery Type
11.3.7.4.6 Australia Spinal Fusion Devices Market, by End User
11.3.7.5 South Korea
11.3.7.5.1 Overview
11.3.7.5.2 South Korea Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.3.7.5.3 South Korea Spinal Fusion Devices Market, by Product Type
11.3.7.5.3.1 South Korea Spinal Fusion Devices Market, by Thoracolumbar Devices
11.3.7.5.3.2 South Korea Spinal Fusion Devices Market, by Cervical Fixation Devices
11.3.7.5.4 South Korea Spinal Fusion Devices Market, by Disease Indication
11.3.7.5.5 South Korea Spinal Fusion Devices Market, by Surgery Type
11.3.7.5.6 South Korea Spinal Fusion Devices Market, by End User
11.3.7.6 Rest of Asia Pacific
11.3.7.6.1 Overview
11.3.7.6.2 Rest of Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.3.7.6.3 Rest of Asia Pacific Spinal Fusion Devices Market, by Product Type
11.3.7.6.3.1 Rest of Asia Pacific Spinal Fusion Devices Market, by Thoracolumbar Devices
11.3.7.6.3.2 Rest of Asia Pacific Spinal Fusion Devices Market, by Cervical Fixation Devices
11.3.7.6.4 Rest of Asia Pacific Spinal Fusion Devices Market, by Disease Indication
11.3.7.6.5 Rest of Asia Pacific Spinal Fusion Devices Market, by Surgery Type
11.3.7.6.6 Rest of Asia Pacific Spinal Fusion Devices Market, by End User
11.4 Middle East & Africa Spinal Fusion Devices Market, Revenue and Forecast To 2030
11.4.1 Overview
11.4.2 Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.4.3 Middle East & Africa Spinal Fusion Devices Market, by Product Type
11.4.3.1 Middle East & Africa Spinal Fusion Devices Market, by Thoracolumbar Devices
11.4.3.2 Middle East & Africa Spinal Fusion Devices Market, by Cervical Fixation Devices
11.4.4 Middle East & Africa Spinal Fusion Devices Market, by Disease Indication
11.4.5 Middle East & Africa Spinal Fusion Devices Market, by Surgery Type
11.4.6 Middle East & Africa Spinal Fusion Devices Market, by End User
11.4.7 Middle East & Africa Spinal Fusion Devices Market, by Country
11.4.7.1 Saudi Arabia
11.4.7.1.1 Overview
11.4.7.1.2 Saudi Arabia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.4.7.1.3 Saudi Arabia Spinal Fusion Devices Market, by Product Type
11.4.7.1.3.1 Saudi Arabia Spinal Fusion Devices Market, by Thoracolumbar Devices
11.4.7.1.3.2 Saudi Arabia Spinal Fusion Devices Market, by Cervical Fixation Devices
11.4.7.1.4 Saudi Arabia Spinal Fusion Devices Market, by Disease Indication
11.4.7.1.5 Saudi Arabia Spinal Fusion Devices Market, by Surgery Type
11.4.7.1.6 Saudi Arabia Spinal Fusion Devices Market, by End User
11.4.7.2 UAE
11.4.7.2.1 Overview
11.4.7.2.2 UAE Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.4.7.2.3 UAE Spinal Fusion Devices Market, by Product Type
11.4.7.2.3.1 UAE Spinal Fusion Devices Market, by Thoracolumbar Devices
11.4.7.2.3.2 UAE Spinal Fusion Devices Market, by Cervical Fixation Devices
11.4.7.2.4 UAE Spinal Fusion Devices Market, by Disease Indication
11.4.7.2.5 UAE Spinal Fusion Devices Market, by Surgery Type
11.4.7.2.6 UAE Spinal Fusion Devices Market, by End User
11.4.7.3 South Africa
11.4.7.3.1 Overview
11.4.7.3.2 South Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.4.7.3.3 South Africa Spinal Fusion Devices Market, by Product Type
11.4.7.3.3.1 South Africa Spinal Fusion Devices Market, by Thoracolumbar Devices
11.4.7.3.3.2 South Africa Spinal Fusion Devices Market, by Cervical Fixation Devices
11.4.7.3.4 South Africa Spinal Fusion Devices Market, by Disease Indication
11.4.7.3.5 South Africa Spinal Fusion Devices Market, by Surgery Type
11.4.7.3.6 South Africa Spinal Fusion Devices Market, by End User
11.4.7.4 Rest of Middle East & Africa
11.4.7.4.1 Overview
11.4.7.4.2 Rest of Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.4.7.4.3 Rest of Middle East & Africa Spinal Fusion Devices Market, by Product Type
11.4.7.4.3.1 Rest of Middle East & Africa Spinal Fusion Devices Market, by Thoracolumbar Devices
11.4.7.4.3.2 Rest of Middle East & Africa Spinal Fusion Devices Market, by Cervical Fixation Devices
11.4.7.4.4 Rest of Middle East & Africa Spinal Fusion Devices Market, by Disease Indication
11.4.7.4.5 Rest of Middle East & Africa Spinal Fusion Devices Market, by Surgery Type
11.4.7.4.6 Rest of Middle East & Africa Spinal Fusion Devices Market, by End User
11.5 South & Central America Spinal Fusion Devices Market, Revenue and Forecast To 2030
11.5.1 Overview
11.5.2 South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.5.3 South & Central America Spinal Fusion Devices Market, by Product Type
11.5.3.1 South & Central America Spinal Fusion Devices Market, by Thoracolumbar Devices
11.5.3.2 South & Central America Spinal Fusion Devices Market, by Cervical Fixation Devices
11.5.4 South & Central America Spinal Fusion Devices Market, by Disease Indication
11.5.5 South & Central America Spinal Fusion Devices Market, by Surgery Type
11.5.6 South & Central America Spinal Fusion Devices Market, by End User
11.5.7 South & Central America Spinal Fusion Devices Market, by Country
11.5.7.1 Brazil
11.5.7.1.1 Overview
11.5.7.1.2 Brazil Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.5.7.1.3 Brazil Spinal Fusion Devices Market, by Product Type
11.5.7.1.3.1 Brazil Spinal Fusion Devices Market, by Thoracolumbar Devices
11.5.7.1.3.2 Brazil Spinal Fusion Devices Market, by Cervical Fixation Devices
11.5.7.1.4 Brazil Spinal Fusion Devices Market, by Disease Indication
11.5.7.1.5 Brazil Spinal Fusion Devices Market, by Surgery Type
11.5.7.1.6 Brazil Spinal Fusion Devices Market, by End User
11.5.7.2 Argentina
11.5.7.2.1 Overview
11.5.7.2.2 Argentina Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.5.7.2.3 Argentina Spinal Fusion Devices Market, by Product Type
11.5.7.2.3.1 Argentina Spinal Fusion Devices Market, by Thoracolumbar Devices
11.5.7.2.3.2 Argentina Spinal Fusion Devices Market, by Cervical Fixation Devices
11.5.7.2.4 Argentina Spinal Fusion Devices Market, by Disease Indication
11.5.7.2.5 Argentina Spinal Fusion Devices Market, by Surgery Type
11.5.7.2.6 Argentina Spinal Fusion Devices Market, by End User
11.5.7.3 Rest of South & Central America
11.5.7.3.1 Overview
11.5.7.3.2 Rest of South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
11.5.7.3.3 Rest of South & Central America Spinal Fusion Devices Market, by Product Type
11.5.7.3.3.1 Rest of South & Central America Spinal Fusion Devices Market, by Thoracolumbar Devices
11.5.7.3.3.2 Rest of South & Central America Spinal Fusion Devices Market, by Cervical Fixation Devices
11.5.7.3.4 Rest of South & Central America Spinal Fusion Devices Market, by Disease Indication
11.5.7.3.5 Rest of South & Central America Spinal Fusion Devices Market, by Surgery Type
11.5.7.3.6 Rest of South & Central America Spinal Fusion Devices Market, by End User
12. Pre & Post Covid-19 Impact
12.1 Pre & Post Covid-19 Impact
13. Spinal Fusion Devices Market Industry Landscape
13.1 Overview
13.2 Growth Strategies in Spinal Fusion Devices Market
13.3 Organic Growth Strategies
13.3.1 Overview
13.4 Inorganic Growth Strategies
13.4.1 Overview
14. Company Profiles
14.1 DePuy Synthes Inc
14.1.1 Key Facts
14.1.2 Business Description
14.1.3 Products and Services
14.1.4 Financial Overview
14.1.5 SWOT Analysis
14.1.6 Key Developments
14.2 Stryker Corp
14.2.1 Key Facts
14.2.2 Business Description
14.2.3 Products and Services
14.2.4 Financial Overview
14.2.5 SWOT Analysis
14.2.6 Key Developments
14.3 B. Braun SE
14.3.1 Key Facts
14.3.2 Business Description
14.3.3 Products and Services
14.3.4 Financial Overview
14.3.5 SWOT Analysis
14.3.6 Key Developments
14.4 ATEC Spine Inc
14.4.1 Key Facts
14.4.2 Business Description
14.4.3 Products and Services
14.4.4 Financial Overview
14.4.5 SWOT Analysis
14.4.6 Key Developments
14.5 Globus Medical Inc
14.5.1 Key Facts
14.5.2 Business Description
14.5.3 Products and Services
14.5.4 Financial Overview
14.5.5 SWOT Analysis
14.5.6 Key Developments
14.6 NuVasive Inc
14.6.1 Key Facts
14.6.2 Business Description
14.6.3 Products and Services
14.6.4 Financial Overview
14.6.5 SWOT Analysis
14.6.6 Key Developments
14.7 ZimVie Inc
14.7.1 Key Facts
14.7.2 Business Description
14.7.3 Products and Services
14.7.4 Financial Overview
14.7.5 SWOT Analysis
14.7.6 Key Developments
14.8 Medtronic Plc
14.8.1 Key Facts
14.8.2 Business Description
14.8.3 Products and Services
14.8.4 Financial Overview
14.8.5 SWOT Analysis
14.8.6 Key Developments
14.9 Centinel Spine LLC
14.9.1 Key Facts
14.9.2 Business Description
14.9.3 Products and Services
14.9.4 Financial Overview
14.9.5 SWOT Analysis
14.9.6 Key Developments
14.10 Orthofix Medical Inc
14.10.1 Key Facts
14.10.2 Business Description
14.10.3 Products and Services
14.10.4 Financial Overview
14.10.5 SWOT Analysis
14.10.6 Key Developments
15. Appendix
15.1 About Us
15.2 Glossary of Terms
List of Tables
Table 1. Spinal Fusion Devices Market Segmentation
Table 2. List of Vendors
Table 3. North America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 4. North America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 5. North America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 6. North America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 7. North America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 8. North America Spinal Fusion Devices Market Revenue and Forecast To 2028 (US$ Mn) – End User
Table 9. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 10. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 11. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 12. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 13. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 14. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 15. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 16. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 17. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 18. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 19. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 20. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 21. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 22. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 23. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 24. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 25. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 26. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 27. Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 28. Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 29. Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 30. Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 31. Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 32. Europe Spinal Fusion Devices Market Revenue and Forecast To 2028 (US$ Mn) – End User
Table 33. UK Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 34. UK Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 35. UK Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 36. UK Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 37. UK Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 38. UK Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 39. Germany Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 40. Germany Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 41. Germany Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 42. Germany Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 43. Germany Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 44. Germany Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 45. France Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 46. France Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 47. France Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 48. France Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 49. France Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 50. France Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 51. Italy Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 52. Italy Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 53. Italy Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 54. Italy Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 55. Italy Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 56. Italy Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 57. Spain Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 58. Spain Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 59. Spain Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 60. Spain Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 61. Spain Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 62. Spain Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 63. Rest of Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 64. Rest of Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 65. Rest of Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 66. Rest of Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 67. Rest of Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 68. Rest of Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 69. Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 70. Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 71. Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 72. Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 73. Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 74. Asia Pacific Spinal Fusion Devices Market Revenue and Forecast To 2028 (US$ Mn) – End User
Table 75. China Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 76. China Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 77. China Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 78. China Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 79. China Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 80. China Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 81. Japan Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 82. Japan Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 83. Japan Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 84. Japan Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 85. Japan Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 86. Japan Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 87. India Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 88. India Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 89. India Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 90. India Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 91. India Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 92. India Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 93. Australia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 94. Australia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 95. Australia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 96. Australia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 97. Australia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 98. Australia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 99. South Korea Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 100. South Korea Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 101. South Korea Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 102. South Korea Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 103. South Korea Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 104. South Korea Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 105. Rest of Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 106. Rest of Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 107. Rest of Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 108. Rest of Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 109. Rest of Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 110. Rest of Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 111. Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 112. Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 113. Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 114. Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 115. Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 116. Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast To 2028 (US$ Mn) – End User
Table 117. Saudi Arabia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 118. Saudi Arabia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 119. Saudi Arabia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 120. Saudi Arabia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 121. Saudi Arabia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 122. Saudi Arabia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 123. UAE Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 124. UAE Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 125. UAE Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 126. UAE Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 127. UAE Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 128. UAE Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 129. South Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 130. South Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 131. South Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 132. South Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 133. South Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 134. South Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 135. Rest of Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 136. Rest of Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 137. Rest of Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 138. Rest of Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 139. Rest of Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 140. Rest of Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 141. South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 142. South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 143. South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 144. South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 145. South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 146. South & Central America Spinal Fusion Devices Market Revenue and Forecast To 2028 (US$ Mn) – End User
Table 147. Brazil Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 148. Brazil Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 149. Brazil Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 150. Brazil Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 151. Brazil Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 152. Brazil Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 153. Argentina Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 154. Argentina Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 155. Argentina Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 156. Argentina Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 157. Argentina Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 158. Argentina Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 159. Rest of South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Product Type
Table 160. Rest of South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Thoracolumbar Devices
Table 161. Rest of South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Cervical Fixation Devices
Table 162. Rest of South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Disease Indication
Table 163. Rest of South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – Surgery Type
Table 164. Rest of South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn) – End User
Table 165. Recent Organic Growth Strategies in Spinal Fusion Devices Market
Table 166. Recent Inorganic Growth Strategies in the Spinal Fusion Devices Market
Table 167. Glossary of Terms, spinal fusion devices market
List of Figures
Figure 1. Spinal Fusion Devices Market Segmentation, By Geography
Figure 2. Global - PEST Analysis
Figure 3. Spinal Fusion Devices Market - Key Industry Dynamics
Figure 4. Impact Analysis of Drivers and Restraints
Figure 5. Spinal Fusion Devices Market Revenue (US$ Mn), 2022 – 2030
Figure 6. Spinal Fusion Devices Market Revenue Share, by Product Type, 2022 & 2030 (%)
Figure 7. Thoracolumbar Devices: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 8. Anterior Lumbar Plates: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 9. Pedicle Screw and Rods: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 10. Others: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 11. Cervical Fixation Devices: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 12. Anterior Cervical Plates: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 13. Hook Fixation Systems: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 14. Others: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 15. Interbody Fusion Devices: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 16. Spinal Fusion Devices Market Revenue Share, by Surgery Type, 2022 & 2030 (%)
Figure 17. Open Spine Surgery: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 18. Minimally Invasive Spine Surgery: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 19. Spinal Fusion Devices Market Revenue Share, by Disease Indications 2022 & 2030 (%)
Figure 20. Degenerative Disc: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 21. Trauma and Fractures: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 22. Complex Deformity: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 23. Others: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 24. Spinal Fusion Devices Market Revenue Share, by End User 2022 & 2030 (%)
Figure 25. Hospitals: Spinal Fusion Devices Market– Revenue and Forecast to 2030 (US$ Million)
Figure 26. Specialty Clinics: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 27. Others: Spinal Fusion Devices Market – Revenue and Forecast to 2030 (US$ Million)
Figure 28. Spinal Fusion Devices Market, 2022 ($Mn)
Figure 29. North America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 30. North America Spinal Fusion Devices Market, By Key Countries, 2022 And 2030 (%)
Figure 31. US Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 32. Canada Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 33. Mexico Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 34. Spinal Fusion Devices Market, 2022 ($Mn)
Figure 35. Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 36. Europe Spinal Fusion Devices Market, By Key Countries, 2022 And 2030 (%)
Figure 37. UK Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 38. Germany Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 39. France Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 40. Italy Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 41. Spain Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 42. Rest of Europe Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 43. Spinal Fusion Devices Market, 2022 ($Mn)
Figure 44. Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 45. Asia Pacific Spinal Fusion Devices Market, By Key Countries, 2022 And 2030 (%)
Figure 46. China Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 47. Japan Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 48. India Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 49. Australia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 50. South Korea Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 51. Rest of Asia Pacific Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 52. Spinal Fusion Devices Market, 2022 ($Mn)
Figure 53. Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 54. Middle East & Africa Spinal Fusion Devices Market, By Key Countries, 2022 And 2030 (%)
Figure 55. Saudi Arabia Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 56. UAE Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 57. South Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 58. Rest of Middle East & Africa Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 59. Spinal Fusion Devices Market, 2022 ($Mn)
Figure 60. South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 61. South & Central America Spinal Fusion Devices Market, By Key Countries, 2022 And 2030 (%)
Figure 62. Brazil Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 63. Argentina Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 64. Rest of South & Central America Spinal Fusion Devices Market Revenue and Forecast to 2030 (US$ Mn)
Figure 65. Pre & Post Covid-19 Impact
Figure 66. Growth Strategies in Spinal Fusion Devices Market
The List of Companies - Spinal Fusion Devices Market
- DePuy Synthes Inc.
- Stryker Corp
- B. Braun SE
- ATEC Spine Inc.
- Globus Medical Inc.
- NuVasive Inc.
- ZimVie Inc.
- Medtronic Plc
- Centinel Spine LLC
- Orthofix Medical Inc.
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
 Get Free Sample For
Get Free Sample For