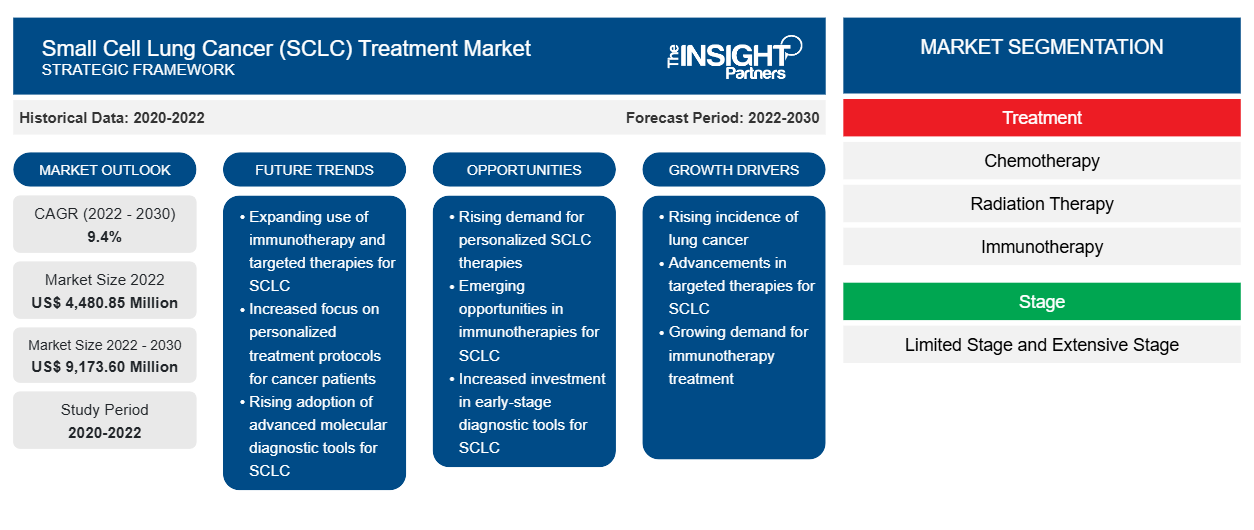
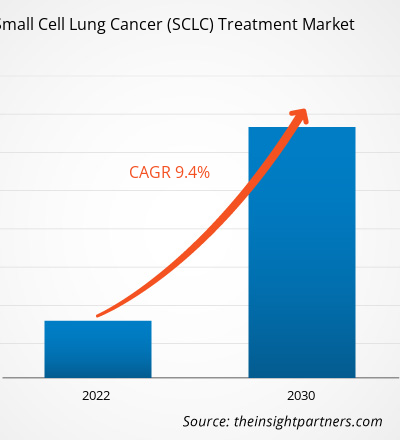
[Research Report] The small cell lung cancer treatment market size is expected to grow from US$ 4,480.85 million in 2022 to US$ 9,173.60 million by 2030; the market is estimated to register a CAGR of 9.4% from 2022 to 2030.
The rising prevalence of SCLC and the availability of therapeutics and combination therapy for the small cell lung cancer treatment market are the noteworthy factors driving the SCLC treatment market growth. Product launches and fast product approvals are also key factors responsible for providing lucrative market opportunities during 2020–2030.
Small cell lung cancer (SCLC) is an aggressive form of lung cancer characterized by a rapid and uncontrolled growth of certain cells in the lungs. The primary risk factor of SCLC is the consumption of tobacco and smoking, with symptoms varying from person to person. The SCLC is broken down into main stages, such as limited-stage disease and extensive-stage disease. The SCLC-affected individuals are more often treated with chemotherapy and radiation therapy, and in the case of early-stage cancer, surgery may be recommended.
Growth Drivers:
Rising Prevalence of SCLC and Availability of Therapeutics
According to the Genentech, Inc. 2023 report, SCLC accounts for approximately 13% of all lung cancer cases with difficulty treating. Therefore, SCLC is the most aggressive type of lung cancer, with a 5-year survival rate. Current therapeutic options for SCLC are limited, and there is an unmet need for novel, effective treatments to improve survival. For example, first-line platinum-based chemotherapy (CT) is a mainstay recommended for the treatment. Also, combination chemotherapy drugs such as "Cisplastin" and" Carboplastin" are the most recommended drugs to use in combination with "Etoposide" to treat SCLC.
The National Cancer Institute report reveals that SCLC accounts for nearly 15% of bronchogenic carcinomas. However, the overall incidence of SCLC in the US has decreased during the past few decades as patients have responded well to chemoradiation. For example, thoracic radiation also improved long-term healthcare outcomes for the patients. Therefore, the rising prevalence of SCLC among the population and the availability of specific therapeutics for the treatment are key factors responsible for the influential growth of the global SCLC market during the forecast period 2020–2030.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Small Cell Lung Cancer (SCLC) Treatment Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Small Cell Lung Cancer (SCLC) Treatment Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Combination Therapy for Small Cell Lung Cancer (SCLC) Treatment Market
Combination therapy for lung cancer treatment is the latest advancement in lung cancer research, with clinical studies revealing patients successfully treated results in overall survival rate for SCLC-suffering patients. Chemotherapy drugs in combination with immunotherapy are one such example of combination therapy intended for SCLC. Atezolizumab (Tecentriq) is an FDA-approved treatment for SCLC used in combination with "carboplatin and etoposide" as a first-line therapy for adults suffering from extensive-stage SCLC. Likewise, clinical researchers have identified a combination of two drugs that results in reducing the mortality rate of SCLC-suffering patients, which is the most aggressive form of lung cancer. With the majority of patients responding positively to SCLC as a first-line treatment through chemotherapy immunotherapy, there are high chances of cancer reoccurring. Therefore, combination therapy, such as chemotherapy drugs, namely, "Topotecan (Hycamtin)" and investigational drug "Berzosertib," is responsible for inhibiting a protein that helps repair damaged DNA caused by SCLC. Thus, the factors above are estimated to favor the market growth during 2020–2030 positively.
Market Opportunity
Product Launches and Fast Product Approvals
The majority of people with SCLC respond to initial treatment with chemotherapy and immunotherapy. However, the cancer usually progresses despite additional treatment, and most of the patients die within weeks or months. Therefore, the fast product approvals will most likely provide a lucrative opportunity for the market to grow exponentially from 2020 to 2030. For instance, in March 2020, the Food and Drug Administration (FDA) announced approval for "Durvalumab" with a combination of "Etoposide" and either "Carboplatin" or "Cisplastin" as a first-line treatment for patients suffering from extensive-stage SCLC. Additionally, in March 2019, the USFDA approved "Tecentriq" to treat SCLC. The newly approved "Tecentriq" is the second immunotherapy drug approved for people with advanced SCLC and a first-line treatment drug.
Future Trend
CAR T Cell Therapy to Provide New Treatment Option
New research approaches to treat SCLC may show great promise in the forthcoming years. CAR-T cell therapy is one example that has revealed a great promise to SCLC in the pre-clinical study. CAR T cell therapy, a "living drug," is responsible for showing pharmacological activity, such as re-engineering a patient's T cells that can attach themselves to cancer cells and destroy them. Delta-like ligand 3 (DLL3), an antigen found on the surface of SCLC tumor cells, includes high-grade cancers of the lung, prostate, breast, pancreas & intestinal tract, and therefore, CAR T cell therapy targeting DDL3 could provide new treatment options for those diseases. Therefore, advancements in CAR T cell therapy will act as a new market trend in the near future ultimately driving the market growth during 2020–2030.
Report Segmentation and Scope:
The global SCLC treatment market is segmented into treatment, stage, and end user. Based on treatment, the market is categorized into chemotherapy, radiation therapy, immunotherapy, and others. In terms of stages, the SCLC treatment market is bifurcated into extensive stage and limited stages. The SCLC treatment market, by the end user, the SCLC treatment market is segmented into hospitals, specialty clinics, home care settings, and others. The SCLC treatment market, based on geography, is segmented into North America (the US, Canada, and Mexico), Europe (Germany, France, Italy, the UK, Spain, and the Rest of Europe), Asia Pacific (Australia, China, Japan, India, South Korea, and the Rest of Asia Pacific), Middle East & Africa (South Africa, Saudi Arabia, the UAE, and the Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Segmental Analysis:
Based on treatment, the SCLC treatment market is segmented into chemotherapy, radiation therapy, immunotherapy, and others. In 2022, the chemotherapy segment held the largest share of the market. Small-cell lung cancer tends to respond well to chemotherapy initially. The chemotherapy drugs for SCLC are usually given through a vein as an intravenous (IV) infusion. Two of the most common chemotherapy drugs for SCLC include "Etoposide Plus Cisplatin" and "Etoposide Plus carboplatin." Additionally, "Topotecan and Lurbinectedin" are chemo drugs that can be taken by patients suffering from SCLC. Therefore, the availability of chemotherapy drugs in combination with the treatment of SCLC is expected to influence the market for the segment during 2020–2030.
The SCLC treatment market based on stage is bifurcated into extensive stage and limited stage. In 2022, the extensive stage segment held the largest share of the market. The same segment is expected to register the highest CAGR in the market during 2022–2030. The extensive stage SCLC spreads widely throughout the lungs, lymph nodes, chest, and other body parts (including bone marrow). As per the American Cancer Society, Inc., 2023 report, about 2 out of 3 people with SCLC have extensive disease in which cancer is first detected. Therefore, with the rising extensive stage of the disease, chemotherapy is the first line treatment option to control the cancer better.
Based on end users, the SCLC treatment market is segmented into hospitals, specialty clinics, homecare settings, and others. In 2022, the hospitals segment held the largest share of the market. However, the specialty clinics segment is expected to register the fastest CAGR during 2022–2030. The SCLC treatment is majorly provided in hospitals as a team of pulmonologists, medical oncologists, radiation oncologists, and thoracic surgeons work closely and create a customized treatment plan for SCLC. The treatment plan includes chemotherapy, radiation therapy, and surgery. Also, hospitals provide multidisciplinary treatments for SCLC and a full range of medical conditions. Also, various support services are provided to improve patients' quality of life and comprehensive and compassionate care. These comprehensive services include pulmonary rehabilitation, smoking cessation, psychological & social support, and integrative therapies. The NYU Langone Hospital is one such example of SCLC therapies. Therefore, customized treatment plans and support services offered by the hospitals are the major factors expected to influence the market growth during 2020–2030.
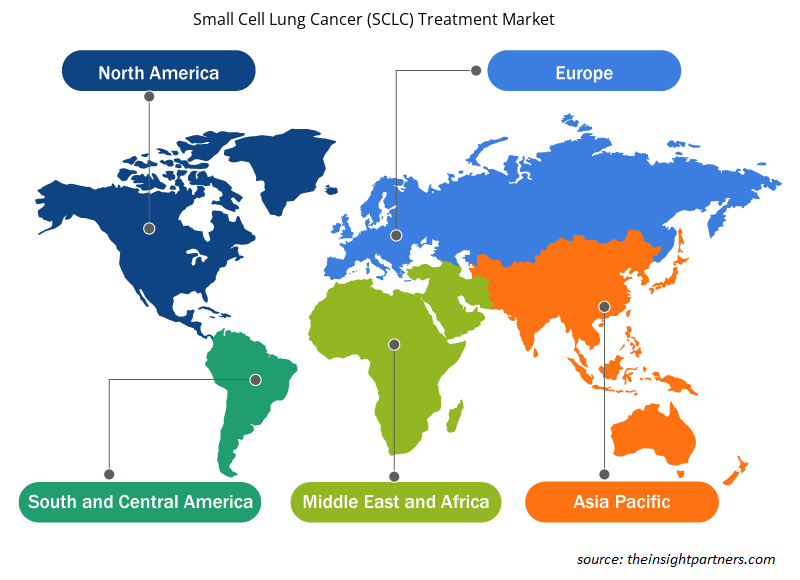
Regional Analysis:
Based on geography, the SCLC treatment market is divided into five key regions: North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. The market in North America has been analyzed with a prime focus on three major countries—the US, Canada, and Mexico. The US held the largest share of the SCLC treatment market in 2022. According to the Yale Medicine 2023 report, SCLC accounts for 15% of all lung cancer diagnoses in the US annually. Therefore, with rising SCLC cases in the US alone, there is a high demand for SCLC drugs to reduce the mortality rate in the US. Likewise, fast product approvals for the drugs to treat SCLC are further expected to enhance the market growth during 2022–2030. For instance, in June 2020, Jazz Pharmaceuticals plc announced USFDA approval for Zepzelcaa" to treat adult patients suffering from metastatic SCLC with disease progression on or after platinum-based chemotherapy. "Zepzelcaa" is now commercially available in the US and will drive market growth from 2020 to 2030.
Likewise, Asia Pacific will register the highest CAGR for the SCLC treatment market during 2022–2030. In Asia Pacific, China holds a major share of the SCLC treatment market during 2020–2030. Fast product approval for the treatment of SCLC in China is a leading factor responsible for the market growth during 2022–2030. For records, in July 2021, AstraZeneca announced approval for "Imfinzi (durvalumab)" in China as a first-line treatment for all adult patients suffering from extensive-stage SCLC. The approval by China's National Medical Products Administration was based on positive results from the CASPIAN Phase III trial, and the clinical trial revealed that "Imfinzi plus" chemotherapy demonstrated a statistically significant and clinically meaningful improvement in the survival rate of patients compared to chemotherapy alone. "Imfinzi Plus" is also being tested following concurrent chemoradiation therapy in patients suffering from limited-stage SCLC in the ADRIATIC Phase III trial as a part of a broad development program. Thus, the factors mentioned above are anticipated to influence the the SCLC treatment market in Asia Pacific during 2020–2030.
Small Cell Lung Cancer (SCLC) Treatment Market Regional Insights
The regional trends and factors influencing the Small Cell Lung Cancer (SCLC) Treatment Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Small Cell Lung Cancer (SCLC) Treatment Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Small Cell Lung Cancer (SCLC) Treatment Market
Small Cell Lung Cancer (SCLC) Treatment Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 4,480.85 Million |
| Market Size by 2030 | US$ 9,173.60 Million |
| Global CAGR (2022 - 2030) | 9.4% |
| Historical Data | 2020-2022 |
| Forecast period | 2022-2030 |
| Segments Covered |
By Treatment
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Small Cell Lung Cancer (SCLC) Treatment Market Players Density: Understanding Its Impact on Business Dynamics
The Small Cell Lung Cancer (SCLC) Treatment Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Small Cell Lung Cancer (SCLC) Treatment Market are:
- F. Hoffmann-La Roche
- Bristol Myers Squibb
- AstraZeneca
- Jazz Pharmaceuticals plc
- Merck KgaA
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Small Cell Lung Cancer (SCLC) Treatment Market top key players overview
Competitive Landscape and Key Companies:
F. Hoffmann-La Roche, Bristol Myers Squibb, AstraZeneca, Jazz Pharmaceuticals plc, Merck KgaA, Dr. Reddy's Laboratories, Inc., GlaxoSmithKline (GSK), Pfizer, Regeneron, and Janssen are a few of the prominent SCLC treatment manufacturers operating in the global SCLC treatment market. These companies focus on new technologies, advancements in existing products, and geographic expansions to meet the increasing consumer demand worldwide and grow their product range in specialty portfolios. In November 2022, Regeneron Pharmaceuticals, Inc. announced approval for the USFDA's "PD-1 inhibitor "Libtayo (cemiplimab-rwlc)" in combination with platinum-based chemotherapy intended for the first-line treatment of adult patients. Libtayo is also approved for extending the survival of patients suffering from advanced SCLC.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Cosmetic Bioactive Ingredients Market
- Sterilization Services Market
- Aircraft Wire and Cable Market
- Health Economics and Outcome Research (HEOR) Services Market
- Dried Blueberry Market
- Radiopharmaceuticals Market
- Maritime Analytics Market
- Small Satellite Market
- Pharmacovigilance and Drug Safety Software Market
- Small Internal Combustion Engine Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Treatment, Stage, End User, and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
The extensive stage segment dominated the global small cell lung cancer (SCLC) treatment market and accounted for the largest revenue in 2022.
F. Hoffmann-La Roche, Bristol Myers Squibb, AstraZeneca, Jazz Pharmaceuticals plc, Merck KgaA, Dr. Reddy's Laboratories, Inc., GlaxoSmithKline (GSK), Pfizer, Regeneron, and Janssen, and among others are among the leading companies operating in the small cell lung cancer (SCLC) treatment market.
Based on treatment, the chemotherapy segment took the forefront lead in the small cell lung cancer (SCLC) treatment market by accounting largest share in 2020 and is expected to continue to do so till the forecast period.
Rising prevalence of SCLC and the availability of therapeutics and combination therapy for the small cell lung cancer treatment market are the factor responsible for the overall market growth.
Small cell lung cancer (SCLC) is an aggressive form of lung cancer characterized by a rapid and uncontrolled growth of certain cells in the lungs. The primary risk factor of SCLC is the consumption of tobacco and smoking, with symptoms varying from person to person. The SCLC is broken down into main stages, such as limited-stage disease and extensive-stage disease. The SCLC-affected individuals are more often treated with chemotherapy and radiation therapy, and in the case of early-stage cancer, surgery may be recommended.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Small Cell Lung Cancer (SCLC) Treatment Market
- F. Hoffmann-La Roche
- Bristol Myers Squibb
- AstraZeneca
- Jazz Pharmaceuticals plc
- Merck KgaA
- Dr. Reddy's Laboratories, Inc.
- GlaxoSmithKline (GSK)
- Pfizer
- Regeneron
- Janssen
 Get Free Sample For
Get Free Sample For