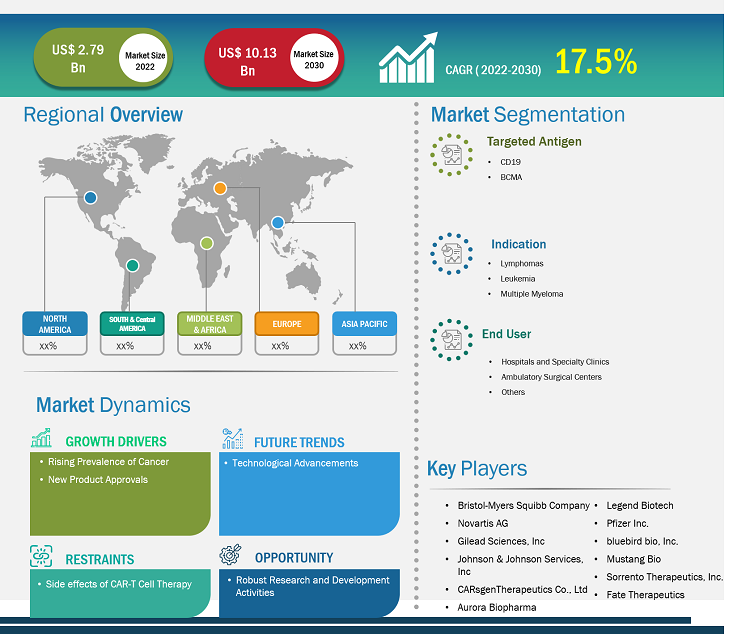
[Research Report] The CAR-T cell therapy market size is projected to reach US$ 10.13billion by 2030 from US$ 2.79 billion in 2022; it is estimated to record a CAGR of 17.5% during 2022–2030.
Market Insights and Analyst View:
One of the most promising methods for treating cancer is chimeric antigen receptor (CAR) T cell therapy, and every year, an enormous number of pre-clinical and clinical trials are conducted to increase its application. After decades of research, CAR-T cell therapy will inevitably expand. In addition to autologous and allogeneic products, new in-vivo CAR-T cell gene therapies and efficacy in indications other than hematologic malignancies will also be investigated in the future of cellular therapies. However, commercializing challenges impede the CAR-T cell therapy market growth.
Growth Drivers:
Rising Prevalence of Cancer Drives CAR-T Cell Therapy Market
CAR-T cell therapy is a new cancer treatment method. T cells are modified in a lab and then returned to the body to target cancer cells. Adult and pediatric leukemia and some types of lymphoma are treated with CAR-T cell therapy. Additionally, it is being researched as a potential treatment for other cancers, such as some solid tumors that develop in the chest.
Genetic predisposition, processed foods, pollution, and altered lifestyles are the factors contributing to the increase in cancer prevalence. According to the American Cancer Society, an estimated 19.3 million new cancer cases were registered globally in 2020. Thus, the increase in the number of patients with cancer is driving the demand for CAR-T cell therapies.
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
CAR-T Cell Therapy Market: Strategic Insights
Market Size Value in US$ 2.79 billion in 2022 Market Size Value by US$ 10.13 billion by 2030 Growth rate CAGR of 17.5% from 2022 to 2030 Forecast Period 2022-2030 Base Year 2022

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
CAR-T Cell Therapy Market: Strategic Insights

| Market Size Value in | US$ 2.79 billion in 2022 |
| Market Size Value by | US$ 10.13 billion by 2030 |
| Growth rate | CAGR of 17.5% from 2022 to 2030 |
| Forecast Period | 2022-2030 |
| Base Year | 2022 |

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Report Segmentation and Scope:
The “CAR-T cell therapy market analysis” has been carried out by considering the following segments: targeted antigen, indication, and end user.
Segmental Analysis:
By targeted antigen, the CAR-T cell therapy market is segmented into CD19 and BCMA. The CD19 segment held a larger CAR-T cell therapy market share in 2022 and is anticipated to register a higher CAGR of 17.9% during the forecast period.
CAR-T cells effectively treat relapsed/refractory diffuse large B cell lymphoma. T cells from patients are genetically modified to produce CD19 CAR T cells. Receptors that bind to the highly expressed CD19 protein found in leukemia and lymphoma cancer cells are expressed by modified T cells.
The availability of CD19 CAR-T cell therapy products is anticipated to drive the segment's growth. Below is the list of products approved by the US Food and Drug Administration and European Union in the US and Europe, respectively:
|
|
|
|
| |
Novartis | KYMRIAH | CD19 | Leukemia | 2017 | |
Gilead Sciences, Inc | YESCARTA | CD19 | Lymphoma | 2017 | |
Gilead Sciences, Inc | TECARTUS | CD19 | Lymphoma & Leukemia | 2020 | |
Bristol Myers Squibb | BREYANZI | CD19 | Lymphoma | 2021 |
The CAR-T cell therapy market, by indication, is divided into lymphomas, leukemia, and multiple myeloma. The lymphomas segment held a larger market share in 2022 and is anticipated to register a higher CAGR of 18.1% during the forecast period. Hematological malignancies such as acute lymphoblastic leukemia, chronic lymphocytic leukemia, lymphoma, and multiple myeloma have been the main indications for the use of CAR-T cells.
The CAR-T cell therapy market, by end user, is categorized into hospitals and specialty clinics, ambulatory surgical centers, and others. The hospitals and specialty clinics segment held the largest market share in 2022 and is anticipated to register the highest CAGR of 17.9% during the forecast period.
Technological Advancements to Accelerate CAR-T Cell Therapy Market Expansion
The creation and introduction of CAR-T therapy, which uses a patient's white blood cells to combat specific forms of blood cancer cells, is among the greatest medical advances of the past few years. Some cancer patients may find hope in CAR-T cell therapy as a potential cure. Five additional CAR-T cell therapies have been approved by the Food and Drug Administration (FDA) since the first therapy was approved in 2017 to treat particular types of adult and pediatric leukemia, lymphoma, and multiple myeloma. Research is also being done to create comparable treatments for solid tumor cancers.
Allogeneic CAR-T cell therapy has been the subject of hundreds of global pre-clinical and clinical trials. Most of these are used for hematological malignancies, where CD19 is the most commonly used target, along with other traditional targets such as CD20, CD22, and BCMA. Included are also newly developed targets such as CD70, CD7, and CD5. Emerging treatments for solid tumors include mesothelin, GD2, and NKG2DL.
Thus, advancements in cell-based therapeutics will likely bring new CAR-T cell therapy market trends in the coming years.
Regional Analysis:
The scope of the global CAR-T cell therapy market report focuses on North America, Europe, Asia Pacific, South & Central America, and the Rest of the World. In 2022, North America held the largest CAR-T cell therapy market share. The market growth in this region is driven by the increasing number of product launches by key manufacturers and the presence of key market players. In addition, extensive R&D by various pharmaceutical and biotechnology companies and academic & research institutes is expected to stimulate market growth in North America. For instance, in June 2022, Bristol Myers Squibb announced receiving the approval of Breyanzi (lisocabtagene maraleucel), a CD19-directed CAR-T cell therapy from the US Food and Drug Administration (FDA) for the treatment of adult patients with large B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL).
In the US, the CAR-T cell therapy market growth is mainly driven by the growing pharmaceutical and biopharmaceutical sector, characterized by technological advancements and increasing flexibility. Furthermore, increasing R&D investments by US-based pharmaceutical and biotechnology companies to improve outcomes of clinical trials and ensure patient safety stimulate market growth. For instance, in July 2021, a purchase agreement was signed by BioNTech SE and Kite, a Gilead Company, allowing BioNTech to purchase Kite's clinical manufacturing facility in Gaithersburg, MD, as well as its solid tumor neoantigen T cell receptor (TCR) R&D platform. The new Gaithersburg location supplements BioNTech's current cell therapy manufacturing facility in Idar-Oberstein, Germany, and offers manufacturing capacity to strengthen clinical trials in the US. The facility helps advance the company's growing pipeline of innovative cell therapies, which includes product candidates for cancer based on NEOSTIM and CAR-T Cell Amplifying mRNA Vaccine (CARVac) and the recently acquired Individualized Neoantigen TCR program.
Future Opportunities and Research and Developments:
CAR-T cell therapies have shown promising outcomes in cancer treatment; nevertheless, their use is restricted to patients with certain liquid tumors that are relapsed and resistant. In the upcoming years, several CAR-T cell therapies for regenerative medicine in the US and advanced therapy medical goods in Europe are anticipated to receive market authorization. Hundreds of different key players with innovative techniques are being developed for a wide range of indications. In the long run, these treatments may improve patient outcomes by offering substantial, even curative, health advantages from a single dosage.
The future of CAR-T cell treatment is quite promising, given the quantity of ongoing clinical trials and new CAR-T cell products under development for a range of indications. Researchers are motivated to investigate ways to enhance the effectiveness of CAR-T cells due to the complete response rates and progression-free survival observed in patients with relapsed/refractory malignancies who had received a lot of prior treatment.
Researchers are looking into elements like antigen loss that could lead to immune escape and are coming up with ways to deal with them. Creating CAR-T cell treatments that simultaneously target several antigens is one such tactic. New medications are being developed to target antigens, such as CD138 and GPRC5D. In contrast, CAR-T cell treatments for ALL and NHL target the CD19 antigen, and therapies for multiple myeloma target the BCMA antigen.
The CAR-T cell therapy market forecast can help stakeholders in this marketplace plan their growth strategies. A few research and developments by leading players operating in the CAR-T cell therapy market are listed below:
- In November 2023, Vittoria Biotherapeutics declared that it completed a private financing round, raising over US$ 15 million. The funds will be utilized to conduct clinical trials of VIPER-101—an autologous dual population CD5-knockout CAR-T cell treatment for T cell lymphoma.
- In December 2023, AstraZeneca announced the acquisition of Gracell Biotechnologies Inc. to complement AstraZeneca’s existing capabilities and previous investments in cell therapy.
- In December 2021, researchers at the University of California San Diego School of Medicine (US) received a US$ 4.1 million grant to support the advancement of their innovative CAR-T cell therapy. The funding, authorized by the governing board of the California Institute for Regenerative Medicine (US), may enable the group to advance novel cancer treatment from the laboratory to the patient's bed.
CAR-T Cell Therapy Market Report Scope
Competitive Landscape and Key Companies:
Bristol-Myers Squibb Company; Novartis AG; Gilead Sciences, Inc.; Johnson & Johnson Services, Inc.; CARsgenTherapeutics Co., Ltd; Aurora Biopharma; Legend Biotech; Pfizer Inc.; bluebird bio, Inc.; Mustang Bio; Sorrento Therapeutics, Inc.; and Fate Therapeutics are among the prominent companies profiled in the CAR-T cell therapy market report. These companies focus on developing new technologies, upgrading existing products, and expanding their geographic presence to meet the growing consumer demand worldwide.

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Targeted Antigen, Indication, End User, and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Chimeric antigen receptor (CAR)-T cell therapy is a kind of cancer immunotherapy treatment that makes use of T cells, which are immune cells, that have undergone genetic alteration in a lab to improve their ability to recognize and eliminate cancer cells. CAR-T cell therapy is one of the newest and most promising treatments for blood cancer.
Key factors that are driving the growth of this market are new product approvals and increase in prevalence of cancer is expected to boost the market growth for the CAR-T cell therapy over the years.
The CD19 segment held the largest share of the market in the global CAR-T cell therapy market and held the largest market share in 2022.
The CAGR value of the CAR-T cell therapy market during the forecasted period of 2022-2030 is 17.5%s
The Lymphomas segment dominated the global CAR-T cell therapy market and held the largest market share in 2022.
Gilead Sciences, Inc and Bristol Myers Squibb are the top two companies that hold huge market shares in the CAR-T cell therapy market.
Global CAR-T cell therapy market is segmented by region into North America, Europe, Asia Pacific, Rest of World. North America held the largest market share of the CAR-T cell therapy market in 2022.
The CAR-T cell therapy market majorly consists of the players such Bristol-Myers Squibb Company, Novartis AG, Gilead Sciences, Inc., Johnson & Johnson Services, Inc., CARsgenTherapeutics Co., Ltd, Aurora Biopharma, Legend Biotech, Pfizer Inc., bluebird bio, Inc., Mustang Bio, Sorrento Therapeutics, Inc., and Fate Therapeutics, and amongst others.
1. Introduction
1.1 Scope of the Study
1.2 Market Definition, Assumptions and Limitations
1.3 Market Segmentation
2. Executive Summary
2.1 Key Insights
2.2 Market Attractiveness Analysis
3. Research Methodology
4. CAR-T Cell Therapy Market Landscape
4.1 Overview
4.2 PEST Analysis
4.3 Ecosystem Analysis
4.3.1 List of Vendors in the Value Chain
5. CAR-T Cell Therapy Market - Key Market Dynamics
5.1 Key Market Drivers
5.2 Key Market Restraints
5.3 Key Market Opportunities
5.4 Future Trends
5.5 Impact Analysis of Drivers and Restraints
6. CAR-T Cell Therapy Market - Global Market Analysis
6.1 CAR-T Cell Therapy - Global Market Overview
6.2 CAR-T Cell Therapy - Global Market and Forecast to 2030
7. CAR-T Cell Therapy Market – Revenue Analysis (USD Billion) – By Targeted Antigen, 2020-2030
7.1 Overview
7.2 CD19
7.3 BCMA
8. CAR-T Cell Therapy Market – Revenue Analysis (USD Billion) – By Indication, 2020-2030
8.1 Overview
8.2 Lymphomas
8.3 Leukemia
8.4 Multiple Myeloma
9. CAR-T Cell Therapy Market – Revenue Analysis (USD Billion) – By End User, 2020-2030
9.1 Overview
9.2 Hospitals and Specialty Clinics
9.3 Ambulatory Surgical Centers
9.4 Others
10. CAR-T Cell Therapy Market - Revenue Analysis (USD Billion), 2020-2030 – Geographical Analysis
10.1 North America
10.1.1 North America CAR-T Cell Therapy Market Overview
10.1.2 North America CAR-T Cell Therapy Market Revenue and Forecasts to 2030
10.1.3 North America CAR-T Cell Therapy Market Revenue and Forecasts and Analysis - By Targeted Antigen
10.1.4 North America CAR-T Cell Therapy Market Revenue and Forecasts and Analysis - By Indication
10.1.5 North America CAR-T Cell Therapy Market Revenue and Forecasts and Analysis - By End User
10.1.6 North America CAR-T Cell Therapy Market Revenue and Forecasts and Analysis - By Countries
10.1.6.1 United States CAR-T Cell Therapy Market
10.1.6.1.1 United States CAR-T Cell Therapy Market, by Targeted Antigen
10.1.6.1.2 United States CAR-T Cell Therapy Market, by Indication
10.1.6.1.3 United States CAR-T Cell Therapy Market, by End User
10.1.6.2 Canada CAR-T Cell Therapy Market
10.1.6.2.1 Canada CAR-T Cell Therapy Market, by Targeted Antigen
10.1.6.2.2 Canada CAR-T Cell Therapy Market, by Indication
10.1.6.2.3 Canada CAR-T Cell Therapy Market, by End User
10.1.6.3 Mexico CAR-T Cell Therapy Market
10.1.6.3.1 Mexico CAR-T Cell Therapy Market, by Targeted Antigen
10.1.6.3.2 Mexico CAR-T Cell Therapy Market, by Indication
10.1.6.3.3 Mexico CAR-T Cell Therapy Market, by End User
Note - Similar analysis would be provided for below mentioned regions/countries
10.2 Europe
10.2.1 Germany
10.2.2 France
10.2.3 Italy
10.2.4 Spain
10.2.5 United Kingdom
10.2.6 Rest of Europe
10.3 Asia-Pacific
10.3.1 Australia
10.3.2 China
10.3.3 India
10.3.4 Japan
10.3.5 South Korea
10.3.6 Rest of Asia-Pacific
10.4 Rest of World
11. Industry Landscape
11.1 Mergers and Acquisitions
11.2 Agreements, Collaborations, Joint Ventures
11.3 New Product Launches
11.4 Expansions and Other Strategic Developments
12. Competitive Landscape
12.1 Heat Map Analysis by Key Players
12.2 Company Positioning and Concentration
13. CAR-T Cell Therapy Market - Key Company Profiles
13.1 Bristol-Myers Squibb Company
13.1.1 Key Facts
13.1.2 Business Description
13.1.3 Products and Services
13.1.4 Financial Overview
13.1.5 SWOT Analysis
13.1.6 Key Developments
Note - Similar information would be provided for below list of companies
13.2 Novartis AG
13.3 Gilead Sciences, Inc.
13.4 Gilead Sciences, Inc.
13.5 Johnson and Johnson Services, Inc
13.6 CARsgen Therapeutics Holdings Limited
13.7 Aurora Biopharma
13.8 Legend Biotech
13.9 Pfizer Inc
13.10 bluebird bio, Inc.
13.11 Mustang Bio
13.12 Sorrento Therapeutics, Inc.
13.13 Fate Therapeutics
14. Appendix
14.1 Glossary
14.2 About The Insight Partners
14.3 Market Intelligence Cloud
The List of Companies - CAR-T Cell Therapy Market
- Bristol-Myers Squibb Company
- Novartis AG
- Gilead Sciences, Inc.
- Johnson & Johnson Services, Inc.
- CARsgenTherapeutics Co., Ltd
- Aurora Biopharma
- Legend Biotech
- Pfizer Inc.
- bluebird bio, Inc.
- Mustang Bio
- Sorrento Therapeutics, Inc
- Fate Therapeutics
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
Trends and growth analysis reports related to CAR-T Cell Therapy Market

Feb 2024
Pediatric Cardiology Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type (Transcatheter Heart Valves, Occlusion Devices, Catheters, Stents, Introducer Sheaths, and Others), Disease Indication (Congenital Heart Disease, Acquired Heart Disease, Arrhythmias, Cardiomyopathies, and Others), Surgical Procedure (Interventional Procedures, Heart Rhythm Management Procedures, and Others), End User (Hospitals, Specialty Clinics, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Feb 2024
Pharmaceutical Membrane Filters Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Technology (Microfiltration, Ultrafiltration, Reverse Osmosis and Nanofiltration), Design (Hollow Fiber, Spiral Wound, Tubular System and Plate and Frame), Material (Polyethersulfone (PES), Polysulfone (PS), Cellulose-Based Membranes, Polytetrafluoroethylene (PTFE), Polyvinyl Chloride (PVC), Polyacrylonitrile (PAN) and Others), End User (Pharmaceutical and Biotech Industries and CROs and CDMOs), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, South & Central America)

Feb 2024
ECG Devices Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Resting ECG and Stress ECG), Lead Type (12-Lead ECG, 3–6 Lead ECG, and Single Lead), Technology [Portable (Wired) ECG System and Wireless ECG System], End User (Hospital and Clinics, Ambulatory Surgical Centers, Cardiac Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Feb 2024
Surgical Laser Fiber Units Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Laser Type (CO2 Laser, Diode Laser, Erbium Laser, Nd:YAG Laser, Holmium Laser, Alexandrite Laser, and Others), Material (Silica-Based Fibers, Quartz Fibers, Polymer-Based Fibers, Multimode Fibers, and Others), Power (Low-Power Lasers, Medium-Power Lasers, and High-Power Lasers), Application (Urology, Dermatology, Gynecology, Cardiology, Neurology, Ophthalmology, Respiratory, Dentistry and Others), Wavelength (9,301 nm and above, 2,941–9,300 nm, and 1,441–2,940 nm, 821–1,440 nm, 710–820 nm, and below 710 nm), End User (Hospitals, Specialty Clinics, Physician Office, and Others), and Geography

Feb 2024
Therapeutic Vaccines Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Cancer Vaccines, Infectious Disease Vaccines, and Others), Technology (Allogenic Vaccines and Autologous Vaccines), End User (Hospitals, Clinics, and Others), and Geography

Feb 2024
Medical Cables Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Disposable Medical Cables, Reusable Medical Cables, and Custom Medical Cables), Applications [Diagnostics (Ultrasound Cables, Endoscopy Cables, Patient Interface Cables, and Others), Motorized Equipment, Patient Monitoring (ECG Cables, SpO2 Cables, NiBP Cables, EEG Cables, and Others), Surgical and Life Support (Fiber Optics, Modular Local Area Network, and Others), and Others], End User (Hospital and Clinics, Diagnostic Laboratories and Imaging Centers, Ambulatory Surgical Centers, and Others), and Geography

Feb 2024
Laser-Assisted ENT Surgeries Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Laser Type (C02 Laser, Nd:YAG Laser, Diode Laser, Blue Laser, KTP Laser, Argon Laser, and Other Laser Types), Surgery Type [Laser Laryngeal Surgery, Laser Endoscopic Sinus Surgery (LESS), Laser-Assisted Uvulopalatoplasty (LAUP), Laser-Assisted Stapedotomy, Laser-Assisted Tonsillectomy and Adenoidectomy, Laser Turbinates Reduction, Transoral Laser Microsurgery (TLM), Nasal Surgery, and Other Surgery Types], End User (Hospitals and Specialty Clinics, Physician Offices, and Other End Users), and Geography (North America, Europe, Asia Pacific, South and Central America, and Middle East and Africa)

Feb 2024
Mobile Cleanroom Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Softwall and Hardwall), End User (Microelectronics Industry, Pharmaceuticals and Biotechnology Industry, Medical Device Manufacturers, and Others), and Geography
 Get Free Sample For
Get Free Sample For