The in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is projected to reach US$ 6,830.99 million in 2028 from US$ 2,957.65 million in 2021; it is estimated to grow at a CAGR of 12.7% from 2021 to 2028.
In silico clinical trials refer to developing patient-specific models to form virtual cohorts for testing the safety and efficacy of new drugs and medical devices. Companies use sophisticated computational modeling and simulation techniques to test their drug candidates in virtual patients before trying them in humans. In silico modeling, also known as computer modeling, allows researchers to simulate behaviors on a computer screen.
The in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented on the basis of organization size, offering, application, clinical indication, end user, and geography. The market, based on geography, is broadly segmented into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America. The report offers insights and a comprehensive analysis of the market, emphasizing parameters such as in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market size, technological advancements, and market dynamics, along with the analysis of the competitive landscape of the globally leading market players.
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market: Strategic Insights
Market Size Value in US$ 2,957.65 Million in 2021 Market Size Value by US$ 6,830.99 Million in 2028 Growth rate CAGR of 12.7% from 2021 to 2028 Forecast Period 2021-2028 Base Year 2021

Naveen
Have a question?
Naveen will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market: Strategic Insights

| Market Size Value in | US$ 2,957.65 Million in 2021 |
| Market Size Value by | US$ 6,830.99 Million in 2028 |
| Growth rate | CAGR of 12.7% from 2021 to 2028 |
| Forecast Period | 2021-2028 |
| Base Year | 2021 |

Naveen
Have a question?
Naveen will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Market Insights
Rising Concerns Over Animal Welfare to Drive In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Growth during Forecast Period
The rapid use of animals in clinical trials is mainly due to the biological similarities between animals and humans. Animals provide adequate human biology and diseases models to yield relevant information, and consequently, animal models offers significant human health benefits. However, using animals for clinical studies is not the only way of drug and devices discovery, as almost every clinical study causes harm to the animal and its progeny in any manner. For instance, in January 2020, the U.S. Department of Agriculture had reported that approximately 300,000 animals were involved in pain-causing experimental activities in just one year.
Moreover, as mentioned by People for the Ethical Treatment of Animals (PETA), each year, more than 100 million animals, including mice, frogs, rabbits, hamsters, and guinea pigs, are killed in US laboratories. The purpose of sacrificing these animals is biology lessons, medical training, curiosity-driven experimentation, and chemical, drug, food, and cosmetics testing. In silico clinical trials offer an effective way of replacing animal anatomies for conducting various experiments and research & development activities, thus, supporting the growth of the in silico trials market. Additionally, rapidly increasing concerns over animal welfare and consistent efforts of human and animal rights and welfare organizations are boosting the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market growth.
Organization Size Insights
Based on organization size, the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is bifurcated into small & medium organizations and large organizations. In 2021, the large organizations segment held a larger market share. Moreover, the small and medium organization segment is expected to register the highest CAGR in the market during 2021–2028 due to the high usage of the in silico trial methods in large research organizations and institutes. However, in the recent era, medium-sized pharmaceutical and biopharmaceutical companies are adopting the in silico model processes to reach their trial processes and increase their efficacy in creating new products.
Offering Insights
Based on offering, the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into products, platforms, and services. In 2021, the products segment held the largest market share. Moreover, the services segment is expected to register the highest CAGR in the market during 2021–2028. The factor attributing to the growth of this segment is low-risk, cost-effective, and virtual environment in in silico trials computational modelling and simulation technology.
Application Insights
Based on application, the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into product design and discovery, product development, pre-clinical targeting, assessment of drugs & other biomedical products, and others. In 2021, the product design and discovery segment held the largest market share. Moreover, the pre-clinical targeting segment is expected to register the highest CAGR in the market during 2021–2028. The factor attributing the growth of this segment is the incorporation of advanced technologies such as machine learning, and artificial intelligence is applied for drug discovery and design applications.
Clinical Indication Insights
Based on clinical indication, the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into cardiovascular diseases, neurodegenerative diseases, oncology, rare diseases, metabolic diseases, immune based diseases, infectious diseases, and others. In 2021, the cardiovascular diseases segment held the largest in silico trials market share. Moreover, the infectious diseases segment is expected to register the highest CAGR in the market during 2021–2028. The factor attributing to the growth of this segment is the rising COVID-19 cases across the globe and strong involvement of the immune system modelling to cure the disease.
End-User Insights
Based on end-users, the global in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented into pharmaceutical and biopharmaceutical companies, medical technology companies, contract research organizations, and others. In 2021, the pharmaceutical and biopharmaceutical companies segment held the largest in silico trials market share. Moreover, the contract research organization segment is expected to register the highest CAGR in the market during 2021–2028.
Product launches, mergers & acquisitions, and collaborations are highly adopted strategies by the global market players. A few of the recent key market developments are listed below:
- In September 2021, Sensyne Health announced that it had launched SENSIGHT, an AI-enabled global data analytics platform for the healthcare and life sciences sectors.
- In February 2022, In SilicoTrials announced that it partnered with IonsGate Preclinical Services Inc (IonsGate) to leverage innovative technology like Modeling and Simulation.
Though the COVID-19 pandemic crisis had a devastating effect on several industries, the in-silico trial market experienced significant growth during this period due to the computer-aided drug discovery method. The demand for in silico trials with the help of computational modeling has witnessed a higher growth rate due to increased R&D activities among researchers and biotechnological and biopharmaceutical companies to limit the spread of the coronavirus disease. The increasing mutation rate of the COVID-19 pandemic has encouraged scientists to discover an effective treatment against the disease. Thus, the significance of research and drug discovery was raised, thereby driving the in silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market growth. The above-stated factors show that the pandemic generated substantial investment opportunities in the market.
In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Segmentation
The global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is segmented on the basis of organization size, offering, application, clinical indication, end user, and geography. Based on organization size, the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is bifurcated into small & medium organizations and large organizations. Based on offering, the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is segmented into products, platforms, and services.
Based on application, the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is segmented into product design and discovery, product development, pre-clinical targeting, assessment of drugs and other biomedical products, and others.
Based on clinical indication, the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is segmented into cardiovascular diseases, neurodegenerative diseases, oncology, rare diseases, metabolic diseases, immune based diseases, infectious diseases, and others.
Based on end-users, the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market is segmented into pharmaceutical and biopharmaceutical companies, medical technology companies, contract research organizations, and others.
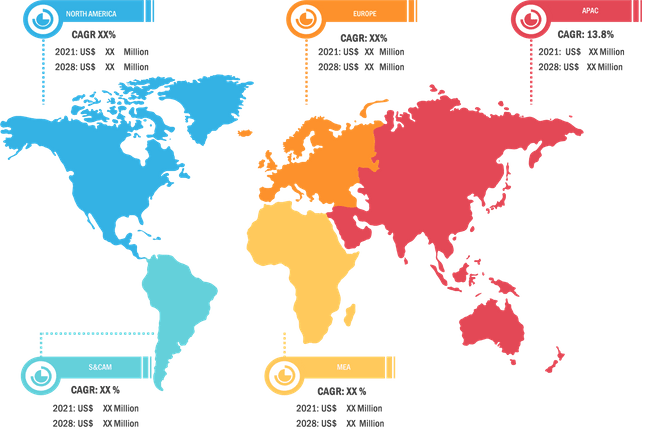
The In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance market, based on geography, is broadly segmented into North America, Europe, Asia Pacific, the Middle East & Africa, and South and Central America. The market in North America is further segmented into the US, Canada, and Mexico. The European market is segmented into France, Germany, the UK, Spain, Italy, and the Rest of Europe.
In Silico Clinical Trials Market Report Scope
The market in Asia Pacific is segmented into China, India, Japan, Australia, South Korea, and the Rest of APAC. The market in the MEA is further segmented into Saudi Arabia, the UAE, South Africa, and the Rest of the MEA. The market in South and Central America is segmented into Brazil, Argentina, and the Rest of South and Central America. InSilico Trials Technologies; FEops; CADFEM Medical GmbH; Dassault Systemes SE; Virtonomy GmbH; Certara Inc.; Computational Life; Novadiscovery; TwInsight Medical; Ansys, Inc.; Synopsys, Inc.; Sensyne Health plc; Phesi; Tempus; and Cerner Corporation are among the leading companies operating in the global market during the forecast period of 2021 to 2028. In May 2020, UK-based Exscientia raised US$60 million in a series C financing round led by Novo Holdings, the wholly-owned holding company of Danish diabetes medicine maker Novo Nordisk, along with German drug development company Evotec, US pharmaceutical company Bristol Myers Squibb, and Asian-based private investment partnership GT Healthcare Capital. This brought the company’s total funding to just over US$100 million. The new capital will be used in part to expand the company’s AI capabilities in biology.

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Organization Size, Offerings, Application, Clinical Indication, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
Global in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market is segmented by region into North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America. In North America, the U.S. is the largest market for in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market. The US is estimated to hold the largest share in the in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market during the forecast period. The presence of top players and favorable regulations related to product approvals coupled with commercializing new products are the contributing factors for the regional growth. Additionally, the increasing number of R&D activities is the key factor responsible for the Asia-Pacific regional growth for in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance accounting fastest growth of the region during the coming years.
InSilico Trials Technologies, Feops, CADFEM Medical GmbH, Dassault Systèmes, Virtonomy GmbH, Certara Inc., Computational Life, NOVA, TwInsight Medical, Ansys, Inc.; Synopsys, Inc; Sensyne Health plc, Phesi, Tempus, Cerner Corporation are among the leading companies operating in the global in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market.
The pharmaceutical and biopharmaceutical companies segment dominated the global in-silico trials: computational modelling and simulation for medical product innovation and regulatory clearance market and accounted for the largest market share in 2021.
In-silico trials refers to the development of patient-specific models to form virtual cohorts for testing the safety and/or efficacy of new drugs and medical devices. Also, with the development of computational modelling and simulation entire imaging including source, object, detection, and image interpretation components intended for R&D, optimization, technology assessment, and regulatory evaluation can be achieved.
Rising concerns over animal welfare and benefits and benefits associated with in-silico trials are the most significant factors responsible for the overall market growth.
Based on organization size, large organizations segment took the forefront lead in the worldwide market by accounting largest share in 2021 and is expected to continue to do so till the forecast period.
1. Introduction
1.1 Scope of the Study
1.2 The Insight Partners Research Report Guidance
1.2.1 Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – By Organization Size
1.2.2 Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – By Offering
1.2.3 Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – By Application
1.2.4 Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – By Clinical Indication
1.2.5 Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – By End User
1.2.6 Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – By Geography
2. Key Takeaways
3. Research Methodology
3.1 Coverage
3.2 Secondary Research
3.3 Primary Research
4. In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Market Landscape
4.1 Overview
4.2 PEST Analysis
4.2.1 North America PEST Analysis
4.2.2 Europe PEST Analysis
4.2.3 Asia Pacific PEST Analysis
4.2.4 Middle East And Africa PEST Analysis
4.2.5 South And Central America PEST Analysis
4.3 Experts Opinion
5. In-Silico Trial Market – Key Market Dynamics
5.1 Market Drivers
5.1.1 Rising Concerns Over Animal Welfare and Benefits
5.1.2 Insufficient Variable Patient Data and Limited Timescale
5.2 Market Restraints
5.2.1 Insufficient Variable Patient Data and Limited Timescale
5.3 Market Opportunities
5.3.1 Growing Applications of In-silico Trials in Radiology and Orthopedic Device Industry
5.4 Future Trends
5.4.1 Adoption of Artificial Intelligence in In-Silico Drug Discovery
5.5 Impact Analysis
6. In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market– Global Analysis
6.1 Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue Forecast and Analysis
6.2 Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue Forecast and Analysis
6.3 Market Positioning of Key Players
7. Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028– by Organization Size
7.1 Overview
7.2 Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2021 & 2028 (%)
7.3 Large Organizations
7.3.1 Overview
7.3.2 Large Organizations: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
7.4 Small and Medium Organizations
7.4.1 Overview
7.4.2 Small and Medium Organizations: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
8. In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 – Offering
8.1 Overview
8.2 Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Share by Segment - 2021 & 2028 (%)
8.3 Products
8.3.1 Overview
8.3.2 Products: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
8.4 Platforms
8.4.1 Overview
8.4.2 Platforms: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
8.5 Services
8.5.1 Overview
8.5.2 Services: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
9. In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 – Application
9.1 Overview
9.2 Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Share by Segment - 2021 & 2028 (%)
9.3 Product Design and Discovery
9.3.1 Overview
9.3.2 Product Design and Discovery: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
9.4 Product Development
9.4.1 Overview
9.4.2 Product Development: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
9.5 Pre-Clinical Targeting
9.5.1 Overview
9.5.2 Pre-Clinical Targeting: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
9.6 Assessment of Drugs and Other Biomedical Products
9.6.1 Overview
9.6.2 Assessment of Drugs and Other Biomedical Products: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
9.7 Others (Optimization and Market Access)
9.7.1 Overview
9.7.2 Others (Optimization and Market Access): In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
10. In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 – Clinical Indication
10.1 Overview
10.2 Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Share by Segment - 2021 & 2028 (%)
10.3 Cardiovascular Disease
10.3.1 Overview
10.3.2 Cardiovascular Disease: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
10.4 Neurodegenerative Disease
10.4.1 Overview
10.4.2 Neurodegenerative Disease: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
10.5 Oncology
10.5.1 Overview
10.5.2 Oncology: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
10.6 Rare Diseases
10.6.1 Overview
10.6.2 Rare Diseases: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
10.7 Metabolic Diseases
10.7.1 Overview
10.7.2 Metabolic Diseases: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
10.8 Immune Based Diseases
10.8.1 Overview
10.8.2 Immune Based Diseases: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
10.9 Infectious Diseases
10.9.1 Overview
10.9.2 Infectious Diseases: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
10.10 Others (Haematology, Diabetes, Dermatology)
10.10.1 Overview
10.10.2 Others (Haematology, Diabetes, and Dermatology): In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
11. In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 – End User
11.1 Overview
11.2 Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Share by Segment - 2021 & 2028 (%)
11.3 Pharmaceutical and Biotechnology Companies
11.3.1 Overview
11.3.2 Pharmaceutical and Biotechnology Companies: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
11.4 Medical Technology Companies
11.4.1 Overview
11.4.2 Medical Technology Companies: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
11.5 Contract Research Organizations
11.5.1 Overview
11.5.2 Contract Research Organizations: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
11.6 Others (Universities and Research Centres)
11.6.1 Overview
11.6.2 Others (Universities and Research Centres): In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
12. In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 – Geographical Analysis
12.1 North America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
12.1.1 Overview
12.1.2 North America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.1.3 North America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size, 2019–2028 (US$ Million)
12.1.4 North America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering, 2019–2028 (US$ Million)
12.1.5 North America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application, 2019–2028 (US$ Million)
12.1.6 North America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (US$ Million)
12.1.7 North America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User, 2019–2028 (US$ Million)
12.1.8 North America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Country, 2021 & 2028 (%)
12.1.8.1 US: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.1.8.1.1 Overview
12.1.8.1.2 US: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.1.8.1.3 US: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size, 2019–2028 (US$ Million)
12.1.8.1.4 US: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering, 2019–2028 (US$ Million)
12.1.8.1.5 US: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application, 2019–2028 (US$ Million)
12.1.8.1.6 US: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (US$ Million)
12.1.8.1.7 US: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User, 2019–2028 (US$ Million)
12.1.8.2 Canada: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.1.8.2.1 Overview
12.1.8.2.2 Canada: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.1.8.2.3 Canada: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size, 2019–2028 (US$ Million)
12.1.8.2.4 Canada: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering, 2019–2028 (US$ Million)
12.1.8.2.4.1 Canada: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application, 2019–2028 (US$ Million)
12.1.8.2.4.2 Canada: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (US$ Million)
12.1.8.2.4.3 Canada: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User, 2019–2028 (US$ Million)
12.1.8.3 Mexico: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.1.8.3.1 Overview
12.1.8.3.2 Mexico: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.1.8.3.3 Mexico: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size, 2019–2028 (US$ Million)
12.1.8.3.4 Mexico: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering, 2019–2028 (US$ Million)
12.1.8.3.4.1 Mexico: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application, 2019–2028 (US$ Million)
12.1.8.3.4.2 Mexico: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (US$ Million)
12.1.8.3.4.3 Mexico: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User, 2019–2028 (US$ Million)
12.2 Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
12.2.1 Overview
12.2.2 Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.3 Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
12.2.4 Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
12.2.4.1 Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.2.4.2 Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.2.4.3 Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.2.5 Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Country, 2021 & 2028 (%)
12.2.5.1 Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.1.1 Overview
12.2.5.1.2 Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.1.3 Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
12.2.5.1.4 Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
12.2.5.1.4.1 Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.2.5.1.4.2 Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.2.5.1.4.3 Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.2.5.2 France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.2.1 Overview
12.2.5.2.2 France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.2.3 France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
12.2.5.2.4 France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
12.2.5.2.4.1 France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.2.5.2.4.2 France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.2.5.2.4.3 France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.2.5.3 UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.3.1 Overview
12.2.5.3.2 UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.3.3 UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
12.2.5.3.4 UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
12.2.5.3.4.1 UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.2.5.3.4.2 UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.2.5.3.4.3 UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.2.5.4 Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.4.1 Overview
12.2.5.4.2 Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.4.3 Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
12.2.5.4.4 Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
12.2.5.4.4.1 Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.2.5.4.4.2 Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.2.5.4.4.3 Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.2.5.5 Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.5.1 Overview
12.2.5.5.2 Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.5.3 Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
12.2.5.5.4 Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
12.2.5.5.4.1 Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.2.5.5.4.2 Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.2.5.5.4.3 Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.2.5.6 Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.6.1 Overview
12.2.5.6.2 Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
12.2.5.6.3 Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
12.2.5.6.4 Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
12.2.5.6.4.1 Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.2.5.6.4.2 Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.2.5.6.4.3 Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.3 Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
12.3.1 Overview
12.3.2 Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market - Revenue and Forecast to 2028 (USD Million)
12.3.3 Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.3.4 Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.3.5 Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.3.6 Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.3.7 Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
12.3.8 Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Country, 2021 & 2028 (%)
12.3.9 China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.9.1 China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.9.2 China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.3.9.3 China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.3.9.4 China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.3.9.5 China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.3.9.6 China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
12.3.10 Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.10.1 Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.10.2 Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.3.10.3 Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.3.10.4 Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.3.10.5 Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.3.10.6 Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
12.3.11 India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.11.1 India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.11.2 India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.3.11.3 India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.3.11.4 India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.3.11.5 India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.3.11.6 India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
12.3.12 Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.12.1 Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.12.2 Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.3.12.3 Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.3.12.4 Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.3.12.5 Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.3.12.6 Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
12.3.13 South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.13.1 South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.13.2 South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.3.13.3 South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.3.13.4 South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.3.13.5 South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.3.13.6 South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
12.3.14 Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.14.1 Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.3.15 Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.3.16 Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.3.17 Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.3.18 Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.3.19 Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
12.4 Middle East & Africa In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028
12.4.1 Overview
12.4.2 Middle East & Africa In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
12.4.3 Middle East & Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Organization Size (US$ Million)
12.4.4 Middle East & Africa: Metabolomics Market Revenue and Forecasts to 2028, By Offering (US$ Million)
12.4.4.1 Middle East & Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.4.4.2 Middle East & Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.4.4.3 Middle East & Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.4.5 Middle East & Africa In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Country (%)
12.4.5.1 UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
12.4.5.1.1 Overview
12.4.5.1.2 UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
12.4.5.1.3 UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Organization Size (US$ Million)
12.4.5.1.4 UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028, By Offering (US$ Million)
12.4.5.1.4.1 UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.4.5.1.4.2 UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.4.5.1.4.3 UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.4.5.2 Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
12.4.5.2.1 Overview
12.4.5.2.2 Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
12.4.5.2.3 Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Organization Size (US$ Million)
12.4.5.2.4 Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028, By Offering (US$ Million)
12.4.5.2.4.1 Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.4.5.2.4.2 Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.4.5.2.4.3 Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.4.5.3 South Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
12.4.5.3.1 Overview
12.4.5.3.2 South Africa In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
12.4.5.3.3 South Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Organization Size (US$ Million)
12.4.5.3.4 South Africa: Metabolomics Market Revenue and Forecasts To 2028, By Offering (US$ Million)
12.4.5.3.4.1 South Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.4.5.3.4.2 South Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.4.5.3.4.3 South Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.4.5.4 Rest of Middle East and Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
12.4.5.4.1 Overview
12.4.5.4.2 Rest of Middle East and Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
12.4.5.4.3 Rest of Middle East & Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Organization Size (US$ Million)
12.4.5.4.4 Rest of Middle East & Africa: Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028, By Offering (US$ Million)
12.4.5.4.4.1 Rest of Middle East and Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
12.4.5.4.4.2 Rest of Middle East and Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
12.4.5.4.4.3 Rest of Middle East and Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
12.5 South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
12.5.1 Overview
12.5.2 South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.5.3 South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.5.4 South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.5.5 South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.5.6 South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.5.7 South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
12.5.8 South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Country, 2021 & 2028 (%)
12.5.9 Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.5.9.1 Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.5.9.2 Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.5.9.3 Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.5.9.4 Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.5.9.5 Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.5.9.6 Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
12.5.10 Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.5.10.1 Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.5.10.2 Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.5.10.3 Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.5.10.4 Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.5.10.5 Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.5.10.6 Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
12.5.11 Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.5.11.1 Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
12.5.11.2 Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size, 2019–2028 (USD Million)
12.5.11.3 Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings, 2019–2028 (USD Million)
12.5.11.4 Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application, 2019–2028 (USD Million)
12.5.11.5 Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication, 2019–2028 (USD Million)
12.5.11.6 Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User, 2019–2028 (USD Million)
13. Impact Of COVID-19 Pandemic on In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
13.1 North America: Impact Assessment of COVID-19 Pandemic
13.2 Europe: Impact Assessment of COVID-19 Pandemic
13.3 Asia-Pacific: Impact Assessment of COVID-19 Pandemic
13.4 Middle East and Africa: Impact Assessment of COVID-19 Pandemic
13.5 South and Central America: Impact Assessment of COVID-19 Pandemic
14. In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market–Industry Landscape
14.1 Overview
14.2 Growth Strategies in the In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market (%)
14.3 Organic Developments
14.3.1 Overview
14.4 Inorganic Developments
14.4.1 Overview
15. Company Profiles
15.1 InSilicoTrials Technologies
15.1.1 Key Facts
15.1.2 Business Description
15.1.3 Products and Services
15.1.4 Financial Overview
15.1.5 SWOT Analysis
15.1.6 Key Developments
15.2 FEops
15.2.1 Key Facts
15.2.2 Business Description
15.2.3 Products and Services
15.2.4 Financial Overview
15.2.5 SWOT Analysis
15.2.6 Key Developments
15.3 CADFEM Medical GmbH
15.3.1 Key Facts
15.3.2 Business Description
15.3.3 Products and Services
15.3.4 Financial Overview
15.3.5 SWOT Analysis
15.3.6 Key Developments
15.4 Dassault Systèmes SE
15.4.1 Key Facts
15.4.2 Business Description
15.4.3 Products and Services
15.4.4 Financial Overview
15.4.5 SWOT Analysis
15.4.6 Key Developments
15.5 Virtonomy GmbH
15.5.1 Key Facts
15.5.2 Business Description
15.5.3 Products and Services
15.5.4 Financial Overview
15.5.5 SWOT Analysis
15.5.6 Key Developments
15.6 Certara Inc.
15.6.1 Key Facts
15.6.2 Business Description
15.6.3 Products and Services
15.6.4 Financial Overview
15.6.5 SWOT Analysis
15.6.6 Key Developments
15.7 Computational Life
15.7.1 Key Facts
15.7.2 Business Description
15.7.3 Products and Services
15.7.4 Financial Overview
15.7.5 SWOT Analysis
15.7.6 Key Developments
15.8 Novadiscovery
15.8.1 Key Facts
15.8.2 Business Description
15.8.3 Products and Services
15.8.4 Financial Overview
15.8.5 SWOT Analysis
15.8.6 Key Developments
15.9 TwInsight Medical
15.9.1 Key Facts
15.9.2 Business Description
15.9.3 Products and Services
15.9.4 Financial Overview
15.9.5 SWOT Analysis
15.9.6 Key Developments
15.10 Ansys, Inc.
15.10.1 Key Facts
15.10.2 Business Description
15.10.3 Products and Services
15.10.4 Financial Overview
15.10.5 SWOT Analysis
15.10.6 Key Developments
15.11 Synopsys, Inc.
15.11.1 Key Facts
15.11.2 Business Description
15.11.3 Products and Services
15.11.4 Financial Overview
15.11.5 SWOT Analysis
15.11.6 Key Developments
15.12 Sensyne Health plc.
15.12.1 Key Facts
15.12.2 Business Description
15.12.3 Products and Services
15.12.4 Financial Overview
15.12.5 SWOT Analysis
15.12.6 Key Developments
15.13 Phesi
15.13.1 Key Facts
15.13.2 Business Description
15.13.3 Products and Services
15.13.4 Financial Overview
15.13.5 SWOT Analysis
15.13.6 Key Developments
15.14 Tempus
15.14.1 Key Facts
15.14.2 Business Description
15.14.3 Products and Services
15.14.4 Financial Overview
15.14.5 SWOT Analysis
15.14.6 Key Developments
15.15 Cerner Corporation
15.15.1 Key Facts
15.15.2 Business Description
15.15.3 Products and Services
15.15.4 Financial Overview
15.15.5 SWOT Analysis
15.15.6 Key Developments
16. Appendix
16.1 About The Insight Partners
16.2 Glossary of Terms
LIST OF TABLES
Table 1. North America In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 2. North America In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (USD Million)
Table 3. North America In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application– Revenue and Forecast to 2028 (US$ Million)
Table 4. North America In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 5. North America In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User– Revenue and Forecast to 2028 (US$ Million)
Table 6. US In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 7. US In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
Table 8. US In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 9. US In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 10. US In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 11. Canada: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 12. Canada In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
Table 13. Canada In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 14. Canada In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 15. Canada In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 16. Mexico: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 17. Mexico In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
Table 18. Mexico In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 19. Mexico In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 20. Mexico In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 21. Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 22. Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
Table 23. Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 24. Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 25. Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 26. Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 27. Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
Table 28. Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 29. Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 30. Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 31. France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 32. France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
Table 33. France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 34. France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 35. France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 36. UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 37. UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
Table 38. UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 39. UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 40. UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 41. Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 42. Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
Table 43. Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 44. Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 45. Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 46. Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 47. Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
Table 48. Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 49. Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 50. Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 51. Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size – Revenue and Forecast to 2028 (US$ Million)
Table 52. Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offering– Revenue and Forecast to 2028 (US$ Million)
Table 53. Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 54. Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 55. Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 56. Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 57. Asia Pacific In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 58. Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 59. Asia Pacific In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 60. Asia Pacific In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 61. China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 62. China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 63. China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 64. China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 65. China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 66. Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 67. Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 68. Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 69. Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 70. Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 71. India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 72. India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 73. India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 74. India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 75. India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 76. Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 77. Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 78. Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 79. Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 80. Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 81. South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 82. South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 83. South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 84. South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 85. South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 86. Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 87. Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 88. Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 89. Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 90. Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 91. Middle East & Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Organization Size (US$ Million)
Table 92. Middle East & Africa: Metabolomics Market Revenue and Forecasts to 2028, By Offering (US$ Million)
Table 93. Middle East & Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 94. Middle East & Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 95. Middle East & Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 96. UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Organization Size (US$ Million)
Table 97. UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Offering (US$ Million)
Table 98. UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 99. UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 100. UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 101. Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Organization Size (US$ Million)
Table 102. Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Offering (US$ Million)
Table 103. Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 104. Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 105. Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 106. South Africa In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Organization Size (US$ Million)
Table 107. South Africa: Metabolomics Market Revenue and Forecasts to 2028, By Offering (US$ Million)
Table 108. South Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 109. South Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 110. South Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 111. Rest of Middle East & Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Organization Size (US$ Million)
Table 112. Rest of Middle East & Africa: Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Offering (US$ Million)
Table 113. Rest of Middle East and Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Application – Revenue and Forecast to 2028 (US$ Million)
Table 114. Rest of Middle East and Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (US$ Million)
Table 115. Rest of Middle East and Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End-User – Revenue and Forecast to 2028 (US$ Million)
Table 116. South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 117. South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 118. South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 119. South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 120. South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 121. Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 122. Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 123. Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 124. Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 125. Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 126. Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 127. Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 128. Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 129. Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 130. Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 131. Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Organization Size – Revenue and Forecast to 2028 (USD Million)
Table 132. Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Offerings – Revenue and Forecast to 2028 (USD Million)
Table 133. Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Application – Revenue and Forecast to 2028 (USD Million)
Table 134. Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Clinical Indication – Revenue and Forecast to 2028 (USD Million)
Table 135. Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by End User– Revenue and Forecast to 2028 (USD Million)
Table 136. Organic Developments in the In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
Table 137. Inorganic Developments in the In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
Table 138. Glossary of Terms
LIST OF FIGURES
Figure 1. In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Segmentation
Figure 2. In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, By Region
Figure 3. Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Overview
Figure 4. Large Organizations Segment Held Largest Share of Organization Size Segment in In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
Figure 5. Asia Pacific is Expected to Show Remarkable Growth During the Forecast Period
Figure 6. In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Geography (US$ Million)
Figure 7. Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market- Leading Country Markets (US$ Million)
Figure 8. Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, Industry Landscape
Figure 9. North America: PEST Analysis
Figure 10. Europe: PEST Analysis
Figure 11. Asia Pacific: PEST Analysis
Figure 12. Middle East And Africa: PEST Analysis
Figure 13. South And Central America: PEST Analysis
Figure 14. Experts Opinion
Figure 15. In-Silico Trial Market: Impact Analysis of Drivers and Restraints
Figure 16. Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market– Revenue Forecast and Analysis – 2020- 2028
Figure 17. Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market– Revenue Forecast and Analysis – 2021 - 2028
Figure 18. Market Positioning of Key Players in Global In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
Figure 19. Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Organization Size, 2021 & 2028 (%)
Figure 20. Large Organizations: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
Figure 21. Small and Medium Organizations: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
Figure 22. Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Share by Segment - 2021 & 2028 (%)
Figure 23. Products: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 24. Platforms: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 25. Services: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 26. Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Share by Segment - 2021 & 2028 (%)
Figure 27. Product Design and Discovery: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 28. Product Development: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 29. Pre-Clinical Targeting: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 30. Assessment of Drugs and Other Biomedical Products: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 31. Others (Optimization and Market Access): In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 32. Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Share by Segment - 2021 & 2028 (%)
Figure 33. Cardiovascular Disease: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 34. Neurodegenerative Disease: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 35. Oncology: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 36. Rare Diseases: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 37. Metabolic Diseases: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 38. Immune Based Diseases: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 39. Infectious Diseases: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 40. Others (Hematology, Diabetes, and Dermatology): In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 41. Global In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Share by Segment - 2021 & 2028 (%)
Figure 42. Pharmaceutical and Biotechnology Companies: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 43. Medical Technology Companies: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 44. Contract Research Organizations: In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 45. Others (Universities and Research Centers): In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts To 2028 (US$ Million)
Figure 46. North America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Key Country – Revenue (2021) (US$ Million)
Figure 47. North America In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
Figure 48. North America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Country, 2021 & 2028 (%)
Figure 49. US: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
Figure 50. Canada: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
Figure 51. Mexico: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
Figure 52. Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Key Country – Revenue (2021) (US$ Million)
Figure 53. Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (US$ Million)
Figure 54. Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Country, 2021 & 2028 (%)
Figure 55. Germany: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
Figure 56. France: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
Figure 57. UK: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
Figure 58. Italy: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
Figure 59. Spain: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
Figure 60. Rest of Europe: In Silico: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (US$ Million)
Figure 61. Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Key Country – Revenue (2021) (USD Million)
Figure 62. Asia Pacific In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (USD Million)
Figure 63. Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Country, 2021 & 2028 (%)
Figure 64. China: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
Figure 65. Japan: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
Figure 66. India: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
Figure 67. Australia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
Figure 68. South Korea: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
Figure 69. Rest of Asia Pacific: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
Figure 70. Middle East & Africa In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue Overview, by Country, 2021 (US$ Million)
Figure 71. Middle East & Africa In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
Figure 72. Middle East & Africa In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028, By Country (%)
Figure 73. UAE: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
Figure 74. Saudi Arabia: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
Figure 75. South Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
Figure 76. Rest of Middle East and Africa: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecasts to 2028 (US$ Million)
Figure 77. South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Key Country – Revenue (2021) (USD Million)
Figure 78. South and Central America In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Revenue and Forecast to 2028 (USD Million)
Figure 79. South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market, by Country, 2021 & 2028 (%)
Figure 80. Brazil: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
Figure 81. Argentina: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
Figure 82. Rest of South and Central America: In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market – Revenue and Forecast to 2028 (USD Million)
Figure 83. Impact of COVID-19 Pandemic in North American Country Markets
Figure 84. Impact of COVID-19 Pandemic in European Country Markets
Figure 85. Impact of COVID-19 Pandemic in Asia Pacific Country Markets
Figure 86. Impact of COVID-19 Pandemic in Middle East and Africa Country Markets
Figure 87. Impact Of COVID-19 Pandemic in South and Central America Country Markets
Figure 88. Growth Strategies in the In-Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market (%)
The List of Companies - In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market
- InSilico Trials Technologies
- Feops
- CADFEM Medical GmbH
- Dassault Systèmes
- Virtonomy GmbH
- Certara Inc.
- Computational Life
- NOVA
- TwInsight Medical
- Ansys, Inc.
- Synopsys, Inc
- Sensyne Health plc
- Phesi
- Tempus
- Cerner Corporation
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
Trends and growth analysis reports related to In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market

Feb 2022
Flight Planning Software Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Component (Software and Services), Deployment (Cloud and On-Premise), Application (Logistics and Cargo, Airport, Private Airlines, Commercial Airlines, Flight School and Training Center, and Military and Defense), and Geography

Feb 2022
Deepfake AI Detection Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Component (Software and Services), Deployment (Cloud and On-Premises), Enterprise Size (Large Enterprises and SMEs), Industry Vertical (Media and Entertainment, BFSI, Government and Politics, Healthcare and Life Sciences, IT and Telecom, Retail and E-Commerce, and Others), and Geography

Feb 2022
Electronic Patient-Reported Outcomes (ePROS) Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Delivery Mode (Cloud Based and On-Premises), Application (Oncology, Respiratory, and Others), End User [Contract Research Organizations (CROs), Pharmaceutical Companies, and Others], and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Feb 2022
Travel and Expense Management Software Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Deployment Mode (On-Premise and Cloud), Organization Size (Large Enterprises and Small and Medium Enterprises), Industry (BFSI, IT and Telecom, Manufacturing, Healthcare, Government and Defense, Retail, Transport and Logistics, and Others), and Geography

Feb 2022
Manufacturing Execution System (MES) Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Component (Software and Services), Services Type (Professional Services and Managed Services), Deployment (Cloud and On-Premise), Organization Size (Large Enterprises and SMEs), License Type (Subscription-Based and Licensed), Sales Channel (Direct Sales and Channel Partners), End User (Discrete Industry and Process Industry), and Geography

Feb 2022
Maritime Analytics Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Component (Software and Services), Deployment (Cloud and On-Premises), Application (Predictive and Prescriptive Analytics, Optimal Route Mapping, Pricing Insights, Vessel Safety and Security, and Others), End User (Commercial and Military), and Geography

Feb 2022
Environmental Consulting Service Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Service Type (Investment Assessment and Auditing, Permitting and Compliance, Project and Information Management, Monitoring and Testing, and Others), Media Type (Water Management, Waste Management, and Others), Vertical (Energy and Utilities, Chemical and Petroleum, Manufacturing and Process Industries, Transportation and Construction Industries, and Others), and Geography

Feb 2022
Data Protection as a Service Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Service Type [Backup as a Service (BaaS), Disaster Recovery as a Service (DRaaS), and Storage as a Service (STaaS)], Deployment (Public Cloud, Hybrid Cloud, and Private Cloud), Enterprise Size (Large Enterprises and SMEs), End Use Industry (IT and Telecom, BFSI, Healthcare, Manufacturing, Retail and E-Commerce, and Others), and Geography
 Get Free Sample For
Get Free Sample For