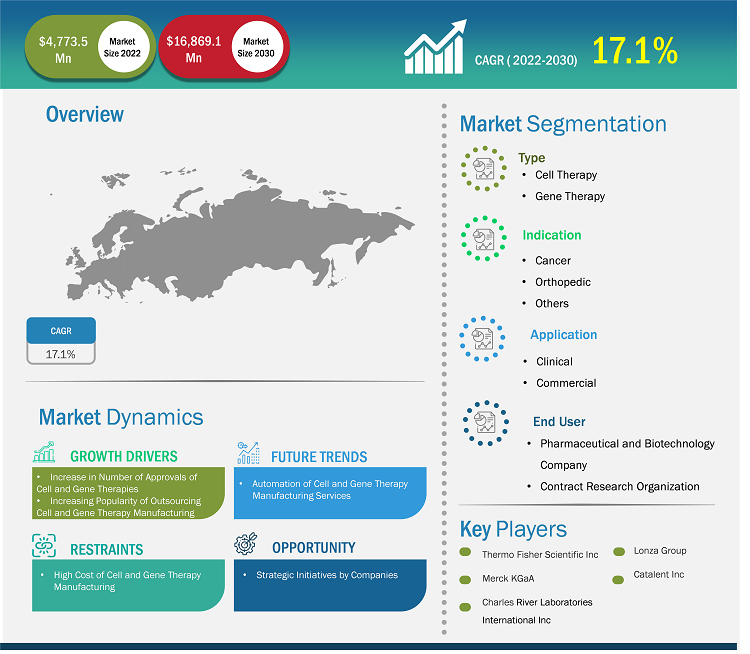
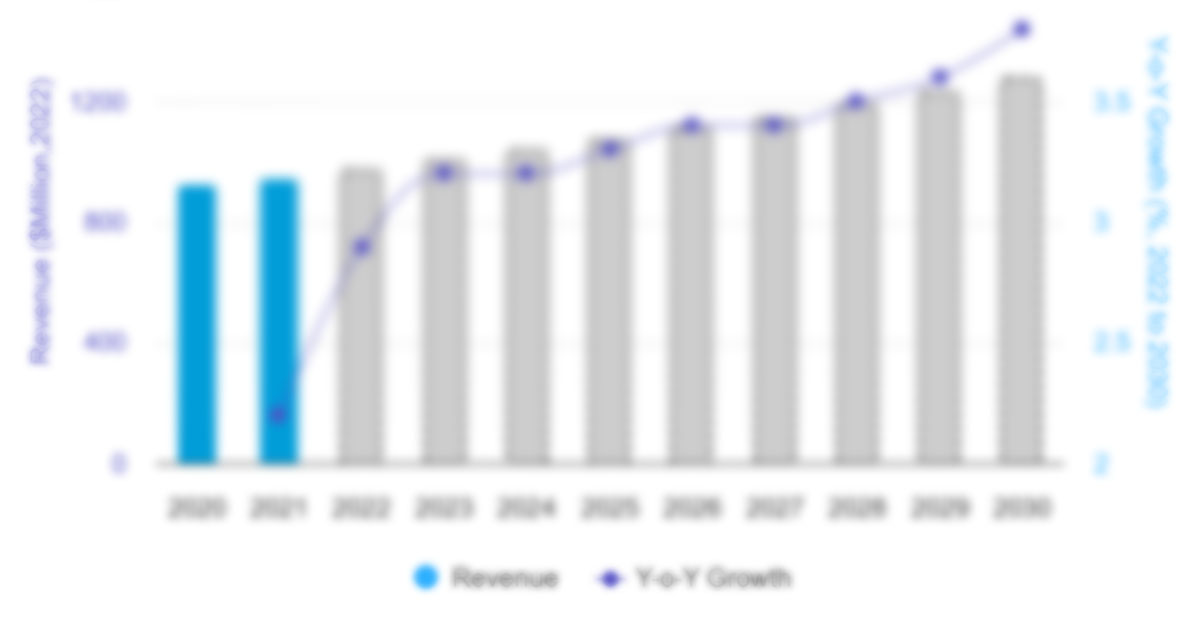
The US and Europe cell and gene therapy manufacturing services market size is expected to grow from US$ 4,773.5 million in 2022 to US$ 16,869.1 million by 2030; it is estimated to register a CAGR of 17.1% from 2022 to 2030.
Analyst’s ViewPoint
The US and Europe cell and gene therapy manufacturing services market aims to support compliance regulations across regions and countries. Once adopted, it provides a comprehensive knowledge-rich environment that enables early detection of rare diseases. Factors such as increase in number of approvals of cell and gene therapies, and increasing popularity of outsourcing cell and gene therapy manufacturing are responsible for the influential growth of the US and Europe cell and gene therapy manufacturing services market. Further, automation of cell and gene therapy manufacturing services acts as a future trend for the market to grow during 2022–2030. According to the segmentation profiled in the report, based on type, the cell therapy segment accounted a maximum share for the US and Europe cell and gene therapy manufacturing services market in 2022. Based on indication, the cancer segment will dominate the market recording maximum share during the forecast period. By application, the commercial manufacturing segment is likely to account considerable share of the cell and gene therapy manufacturing services during the forecast period. In terms of end user, pharmaceutical and biotechnology companies will account a maximum share of the US and Europe cell and gene therapy manufacturing services market during 2022–2030.
Establishing a robust, repeatable, and sustainable process help accelerate development, avoiding manufacturing transfer-related delays. Cell & gene therapy (CGT) programs are rapidly advancing from R&D to clinical trials and commercial approval. The cell and gene therapy comprises the next generation of life-enhancing and curative therapies. With new approvals for these therapies, the demand for skilled professionals in cell and gene therapy manufacturing services would rise in the future, which would encourage more pharmaceutical and biotech companies in the US and Europe to outsource cell and gene therapy manufacturing operations
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
US and Europe Cell and Gene Therapy Manufacturing Services Market: Strategic Insights
Market Size Value in US$ 4,773.5 million in 2022 Market Size Value by US$ 16,869.1 million by 2030 Growth rate CAGR of 17.1% from 2022 to 2030 Forecast Period 2022-2030 Base Year 2022

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
US and Europe Cell and Gene Therapy Manufacturing Services Market: Strategic Insights

| Market Size Value in | US$ 4,773.5 million in 2022 |
| Market Size Value by | US$ 16,869.1 million by 2030 |
| Growth rate | CAGR of 17.1% from 2022 to 2030 |
| Forecast Period | 2022-2030 |
| Base Year | 2022 |

Mrinal
Have a question?
Mrinal will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Market Insights
Increase in Number of Approval of Cell and Gene Therapies Accounts Maximum Share
The advancements in biotechnology have led to the adoption of personalized treatments for a wide range of indications. Stem cell therapies are being used to treat chronic diseases, such as cancer, neurological disorders, and genetic disorders. Further, the advantages of cell therapy, such as targeted treatment, faster and efficient recovery, and reduced side effects, promote the adoption of various products. In US and Europe, cell therapies are widely adopted owing to the availability of Food and Drug Administration (FDA) approved products. Following is the list of cell and gene therapy products approved by the FDA in recent years:
- In April 2020, the FDA awarded regenerative medicine advanced therapy designation to Novartis' Kymriah to treat refractory (r/r) follicular lymphoma (FL) in adults.
- In July 2020, the FDA approved a CAR T-cell therapy brexucabtagene autoleucel (Tecartus) for patients with mantle cell lymphoma. It is the first FDA-approved CAR T-cell therapy for mantle cell lymphoma, and it was approved under the Accelerated Approval pathway. Tecartus also received Orphan Drug designation, which encourages the development of drugs for rare diseases. The other approved CAR-T cell therapies for cancer are Kymriah for acute lymphoblastic leukemia and Yescarta for diffuse large B-cell lymphoma.
- In 2022, ADSTILADRIN, an adenovirus manufactured by Ferring Pharmaceuticals A/S, was approved by FDA. This recombinant adenovirus (rAd-IFNa/Syn3) delivers human interferon alfa-2b cDNA into the bladder epithelium to treat patients with certain types of bladder cancer.
- In 2022, CARVYKTI, manufactured by Janssen Biotech, Inc.—an autologous CAR-T cell engineered with lentivirus to attack BCMA-expressing tumor cells for treatment of certain kinds of relapsed or refractory multiple myeloma—was also approved by the FDA.
- In 2022, the FDA approved HEMGENIX, manufactured by CSL Behring LLC, which is a recombinant AAV5 that delivers F9 to treat patients with certain kinds of Hemophilia B.
- In 2023, the FDA approved VYJUVEK, manufactured by Krystal Biotech, Inc., to treat wounds in patients 6 months of age and older with dystrophic epidermolysis bullosa with mutation(s) in the collagen type VII alpha 1 chain (COL7A1) gene.
Therefore, the increasing approval of cell and gene therapies helps the cell and gene therapy manufacturing services market grow considerably.
Report Segmentation and Scope
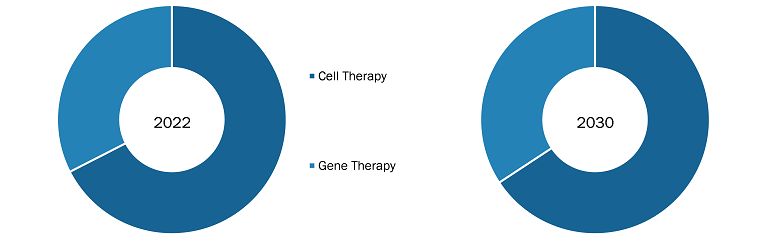
The “US & Europe Cell & Gene Therapy Manufacturing Services Market” is segmented based on type, indication, application, end user, and region. Based on the type, the US & Europe Cell & Gene Therapy Manufacturing Services market is segmented into cell therapy and gene therapy. Based on indication, the US & Europe cell & gene therapy manufacturing services market is segmented into cancer, orthopedics, and others. Based on application, the market is bifurcated into clinical and commercial. By end user, the market is segmented into pharmaceutical & biotechnology companies and contract research organizations (CROs).
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
- Request discounts available for Start-Ups & Universities
Application-Based Insights
In terms of application, the cell and gene therapy manufacturing services market is bifurcated into clinical manufacturing and commercial manufacturing. The commercial manufacturing segment held a larger share of cell and gene therapy manufacturing services in 2022 and is anticipated to register a higher CAGR of 17.9% during the forecast period (2022–2030). CGTs provide significant value across multiple therapeutic areas, driven by a combination of clinical benefit, durability, and overall response rate. However, these therapies are yet to gain a significant market reach. The therapeutic gain of CGTs is underestimated due to the difficulty associated with delivering the right therapy to the right patients, and thus, only a few commercial CGTs-based products have reached the market over the past decade. However, the clinical pipeline of CGT-based products in Phase 3 clinical trials indicates the possibility of a dramatic rise in the number of approvals in the near future.
WuXi Advanced Therapies Inc., a prominent CDMO and a wholly subsidiary of WuXi AppTec, announced the opening of new process development and commercial manufacturing facility in Shanghai in October 2021. This new manufacturing facility would help it expand its global capacity through GMP commercial manufacturing and integrated testing services that would support the commercialization of CGT products in the coming years. The aforementioned factors will be responsible for segmental growth ultimately driving the US and Europe cell and gene therapy manufacturing services market growth during the forecast period.
Cell and Gene Therapy Manufacturing Services Market, by Type – 2022 and 2030
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
- Request discounts available for Start-Ups & Universities
Regional Analysis
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
- Request discounts available for Start-Ups & Universities
The US dominated the cell and gene therapy manufacturing services market accounting maximum share. Cell and gene therapies (CGTs) treat patients suffering from serious and rare diseases with unaddressed therapeutic needs. Manufacturing CGTs is a highly complex process, with the insufficiency of infrastructure and expertise being a major limiting factor. Logistical challenges associated with intermediates and the final product also limit the CGT manufacturing capacity of companies. The CGT manufacturing process involves the extraction of autologous cells through "apheresis," dispatching them to specialized labs, and sending them back to clinics for administration into patients, all of which must be performed with strict quality control. The US Food and Drug Administration (USFDA) has approved only 7 CGT drugs, with the pipeline of new products reaching ~1,200 experimental therapies. Half of these are in Phase 2 clinical trials, with estimates of annual sales growth accounting for 15% for cell therapies and ~30% for gene therapies, as per the estimates of the Chemical & Engineering News report 2023.
Many manufacturers approach contract development manufacturing organizations (CDMOs) such as Labcorp, Lonza, and Catalent to overcome the barriers associated with the production and commercialization of their cell and gene therapy products. Lonza has invested ~US$ 9.2 million to strengthen its cell and gene therapy manufacturing capabilities and support. Such initiatives by CDMOs are contributing to the growth of the cell and gene therapy market in the US.
The report profiles leading players operating in the US and Europe cell and gene therapy manufacturing services market. These include Thermo Fisher Scientific Inc, Merck KGaA, Charles River Laboratories International Inc, Lonza Group AG, WuXi AppTec Co Ltd, Catalent Inc, Takara Bio Inc, Nikon Corp, FUJIFILM Holdings Corp, National Resilience Inc, and Oxford BioMedica Plc.
In April 2022, Erytech Pharma announced acquisition of Catalent acquired from its state-of-the-art, commercial-scale cell therapy manufacturing facility in Princeton, New Jersey, for US$44.5 million. The deal includes an exclusive long-term supply agreement for Catalent to support Erytech’s lead product candidate, eryaspase (GRASPA), a red blood cell-derived product that is currently in late-stage development to treat acute lymphoblastic leukemia. It will also collaborate with Catalent’s existing US clinical-scale cell therapy facility in Houston, Texas.
In January 2022, FUJIFILM Corporation entered into an agreement to acquire a cell therapy manufacturing facility from Atara Biotherapeutics, Inc. for US$ 100 million. The facility is readily expandable with the flexibility to produce both clinical and commercial cell therapies, including allogeneic T-cell and CAR T immunotherapies. As part of the agreement, FUJIFILM and Atara will enter into a long-term manufacturing and services agreement, extending to 10 years to support the production of Atara’s clinical pipeline products.
Company Profiles
- Thermo Fisher Scientific Inc
- Merck KGaA
- Charles River Laboratories International Inc
- Lonza Group AG
- WuXi AppTec Co Ltd
- Catalent Inc
- Takara Bio Inc
- Nikon Corp
- FUJIFILM Holdings Corp
- National Resilience Inc
- Oxford BioMedica Plc

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Type, Indication, Application, and End User, and Country

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Companies operating in the market are Thermo Fisher Scientific Inc, Merck KGaA, Charles River Laboratories International Inc, Lonza Group AG, WuXi AppTec Co Ltd, Catalent Inc, Takara Bio Inc, Nikon Corp, FUJIFILM Holdings Corp, National Resilience Inc, and Oxford BioMedica Plc.
US and Europe Cell and Gene Therapy Manufacturing Services market is segmented by region into Europe, and the Middle East & Africa. US is likely to continue its dominance in the US and Europe Cell and gene therapy manufacturing services market during 2022–2030. The US holds the largest share of the market and is expected to continue this trend during the forecast period.
Major factors driving the market growth includes increase in number of approvals of cell and gene therapies and increasing popularity of outsourcing cell and gene therapy manufacturing. Additionally, automation of cell and gene therapy manufacturing services are likely to emerge as significant future trends in the market during the forecast period.
US and Europe Cell and Gene Therapy Manufacturing Services market, based on type, is segmented into cell therapy and gene therapy. Cell therapy is segmented as autologous and allogenic. Further, gene therapy is bifurcated into viral vector and non-viral vector. The cell therapy segment held a larger market share in 2022. However, gene therapy segment is anticipated to register the highest CAGR during the forecast period (2022-2030).
Answer:- Cell & gene therapy (CGT) programs are rapidly advancing from research & development to clinical trials and commercial approval. Establishing a robust, repeatable, and sustainable process help accelerate development, avoiding manufacturing transfer-related delays. Additionally, cell and gene therapy comprises the next generation of life-enhancing and curative therapies. With therapies experiencing new approvals, the demand for skilled professionals in cell and gene therapy manufacturing services will rise.
Based on indication, the US and Europe cell and gene therapy manufacturing services market is divided into cancer, orthopedics, and others. The cancer segment held the largest share of the market in 2022 and same segment is expected to grow at the highest CAGR during the forecast period.
1. Introduction
1.1 Scope of the Study
1.2 The Insight Partners Research Report Guidance
1.3 Market Segmentation
1.3.1 US and Europe Cell and Gene Therapy Manufacturing Services Market – by Type
1.3.2 US and Europe Cell and Gene Therapy Manufacturing Services Market – by Indication
1.3.3 US and Europe Cell and Gene Therapy Manufacturing Services Market – by Application
1.3.4 US and Europe Cell and Gene Therapy Manufacturing Services Market – by End User
1.3.5 US and Europe Cell and Gene Therapy Manufacturing Services Market – by Country & Region
2. US and Europe Cell and Gene Therapy Manufacturing Services Market – Key Takeaways
3. Research Methodology
3.2 Coverage
3.3 Secondary Research
3.4 Primary Research
4. US and Europe Cell and Gene Therapy Manufacturing Services Market – Market Landscape
4.1 Overview
4.2 PEST Analysis
4.2.1 US PEST Analysis
4.2.2 Europe PEST Analysis
4.3 Expert’s Opinion
5. US and Europe Cell and Gene Therapy Manufacturing Services Market – Key Market Dynamics
5.1 Market Drivers
5.1.1 Increase in Number of Approval of Cell and Gene Therapies
5.1.2 Increasing Popularity of Outsourcing Cell and Gene Therapy Manufacturing
5.2 Market Restraints
5.2.1 High Cost of Cell and Gene Therapy Manufacturing
5.3 Market Opportunities
5.3.1 Strategic Initiatives by Companies
5.4 Future Trends
5.4.1 Automation of Cell and Gene Therapy Manufacturing Services
5.5 Impact Analysis
6. US and Europe Cell and Gene Therapy Manufacturing Services Market – Country & Regional Analysis
6.1 US and Europe Cell and Gene Therapy Manufacturing Services Market Revenue Forecast and Analysis
6.2 Market Positioning of Key Players
7. US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 – by Type
7.1 Overview
7.2 US and Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Type 2022 & 2030 (%)
7.3 Cell Therapy
7.3.1 Overview
7.3.2 Cell Therapy: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
7.3.2.1 Autologous
7.3.2.1.1 Overview
7.3.2.1.2 Autologous: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
7.3.2.2 Allogenic
7.3.2.2.1 Overview
7.3.2.2.2 Allogenic: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
7.4 Gene Therapy
7.4.1 Overview
7.4.2 Gene Therapy: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
7.4.2.1 Viral Vector
7.4.2.1.1 Overview
7.4.2.1.2 Viral Vector: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
7.4.2.2 Non-Viral Vector
7.4.2.2.1 Overview
7.4.2.2.2 Non-Viral Vector: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
8. US and Europe Cell and Gene Therapy Manufacturing Services Market Analysis and Forecasts to 2030 – by Indication
8.1 Overview
8.2 US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication 2022 & 2030 (%)
8.3 Cancer
8.3.1 Overview
8.3.2 Cancer: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
8.4 Orthopedics
8.4.1 Overview
8.4.2 Orthopedics: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
8.5 Others
8.5.1 Overview
8.5.2 Others: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
9. US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 – by Application
9.1 Overview
9.2 US and Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Application 2022 & 2030 (%)
9.4 Clinical Manufacturing
9.4.1 Overview
9.4.2 Clinical Manufacturing: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
9.5 Commercial Manufacturing
9.5.1 Overview
9.5.2 Commercial Manufacturing: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
10. US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 – by End User
10.1 Overview
10.2 US and Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by End User 2022 & 2030 (%)
10.4 Pharmaceutical and Biotechnology Companies
10.4.1 Overview
10.4.2 Pharmaceutical & Biotechnology Companies: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
10.5 Contract Research Organizations (CROs)
10.5.1 Overview
10.5.2 Contract Research Organizations (CROs): US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11. US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 – Country & Regional Analysis
11.1 US: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.1.1 Overview
11.1.2 US: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.1.2.1 US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020–2030 (US$ Million)
11.1.2.1.1 US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020–2030 (US$ Million)
11.1.2.1.2 US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020–2030 (US$ Million)
11.1.2.2 US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020–2030 (US$ Million)
11.1.2.3 US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020–2030 (US$ Million)
11.1.2.4 US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020–2030 (US$ Million)
11.2 Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market
11.2.1 Overview
11.2.2 Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.3 Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020–2030 (US$ Million)
11.2.3.1 Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020–2030 (US$ Million)
11.2.3.2 Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020–2030 (US$ Million)
11.2.4 Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020–2030 (US$ Million)
11.2.5 Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020–2030 (US$ Million)
11.2.6 Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020–2030 (US$ Million)
11.2.7 Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Country, 2022 & 2030 (%)
11.2.7.1 Germany: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.1.1 Overview
11.2.7.1.2 Germany: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.1.3 Germany: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020–2030 (US$ Million)
11.2.7.1.3.1 Germany: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020–2030 (US$ Million)
11.2.7.1.3.2 Germany: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020–2030 (US$ Million)
11.2.7.1.4 Germany: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020–2030 (US$ Million)
11.2.7.1.5 Germany: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020–2030 (US$ Million)
11.2.7.1.6 Germany: US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020–2030 (US$ Million)
11.2.7.2 UK: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.2.1 Overview
11.2.7.2.2 UK: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.2.3 UK: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020–2030 (US$ Million)
11.2.7.2.3.1 UK: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020–2030 (US$ Million)
11.2.7.2.3.2 UK: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020–2030 (US$ Million)
11.2.7.2.4 UK: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020–2030 (US$ Million)
11.2.7.2.5 UK: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020–2030 (US$ Million)
11.2.7.2.6 UK: US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020–2030 (US$ Million)
11.2.7.3 France: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.3.1 Overview
11.2.7.3.2 France: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.3.3 France: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020–2030 (US$ Million)
11.2.7.3.3.1 France: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020–2030 (US$ Million)
11.2.7.3.3.2 France: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020–2030 (US$ Million)
11.2.7.3.4 France: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020–2030 (US$ Million)
11.2.7.3.5 France: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020–2030 (US$ Million)
11.2.7.3.6 France: US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020–2030 (US$ Million)
11.2.7.4 Italy: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.4.1 Overview
11.2.7.4.2 Italy: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.4.3 Italy: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020–2030 (US$ Million)
11.2.7.4.3.1 Italy: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020–2030 (US$ Million)
11.2.7.4.3.2 Italy: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020–2030 (US$ Million)
11.2.7.4.4 Italy: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020–2030 (US$ Million)
11.2.7.4.5 Italy: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020–2030 (US$ Million)
11.2.7.4.6 Italy: US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020–2030 (US$ Million)
11.2.7.5 Spain: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.5.1 Overview
11.2.7.5.2 Spain: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.5.3 Spain: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020–2030 (US$ Million)
11.2.7.5.3.1 Spain: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020–2030 (US$ Million)
11.2.7.5.3.2 Spain: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020–2030 (US$ Million)
11.2.7.5.4 Spain: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020–2030 (US$ Million)
11.2.7.5.5 Spain: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020–2030 (US$ Million)
11.2.7.5.6 Spain: US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020–2030 (US$ Million)
11.2.7.6 Rest of Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.6.1 Overview
11.2.7.6.2 Rest of Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
11.2.7.6.3 Rest of Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020–2030 (US$ Million)
11.2.7.6.3.1 Rest of Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020–2030 (US$ Million)
11.2.7.6.3.2 Rest of Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020–2030 (US$ Million)
11.2.7.6.4 Rest of Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020–2030 (US$ Million)
11.2.7.6.5 Italy: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020–2030 (US$ Million)
11.2.7.6.6 Rest of Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020–2030 (US$ Million)
12. US and Europe Cell and Gene Therapy Manufacturing Services Market – Industry Landscape
12.1 Overview
12.2 Growth Strategies in US and Europe Cell and Gene Therapy Manufacturing Services Market
12.3 Organic Growth Strategies
12.3.1 Overview
12.4 Inorganic Growth Strategies
12.4.1 Overview
13. Company Profiles
13.1 Thermo Fisher Scientific Inc
13.1.1 Key Facts
13.1.2 Business Description
13.1.3 Products and Services
13.1.4 Financial Overview
13.1.5 SWOT Analysis
13.1.6 Key Developments
13.2 Merck KGaA
13.2.1 Key Facts
13.2.2 Business Description
13.2.3 Products and Services
13.2.4 Financial Overview
13.2.5 SWOT Analysis
13.2.6 Key Developments
13.3 Charles River Laboratories InternationalInc
13.3.1 Key Facts
13.3.2 Business Description
13.3.3 Products and Services
13.3.4 Financial Overview
13.3.5 SWOT Analysis
13.3.6 Key Developments
13.4 Lonza Group AG
13.4.1 Key Facts
13.4.2 Business Description
13.4.3 Products and Services
13.4.4 Financial Overview
13.4.5 SWOT Analysis
13.4.6 Key Developments
13.5 WuXi AppTec Co Ltd?
13.5.1 Key Facts
13.5.2 Business Description
13.5.3 Products and Services
13.5.4 Financial Overview
13.5.5 SWOT Analysis
13.5.6 Key Developments
13.6 Catalent Inc
13.6.1 Key Facts
13.6.2 Business Description
13.6.3 Products and Services
13.6.4 Financial Overview
13.6.5 SWOT Analysis
13.6.6 Key Developments
13.7 Takara Bio Inc
13.7.1 Key Facts
13.7.2 Business Description
13.7.3 Products and Services
13.7.4 Financial Overview
13.7.5 SWOT Analysis
13.7.6 Key Developments
13.8 Nikon Corp
13.8.1 Key Facts
13.8.2 Business Description
13.8.3 Products and Services
13.8.4 Financial Overview
13.8.5 SWOT Analysis
13.8.6 Key Developments
13.9 FUJIFILM Holdings Corp
13.9.1 Key Facts
13.9.2 Business Description
13.9.3 Products and Services
13.9.4 Financial Overview
13.9.5 SWOT Analysis
13.9.6 Key Developments
13.10 National Resilience Inc
13.10.1 Key Facts
13.10.2 Business Description
13.10.3 Products and Services
13.10.4 Financial Overview
13.10.5 SWOT Analysis
13.10.6 Key Developments
13.11 Oxford BioMedica Plc
13.11.1 Key Facts
13.11.2 Business Description
13.11.3 Products and Services
13.11.4 Financial Overview
13.11.5 SWOT Analysis
13.11.6 Key Developments
14. Appendix
14.1 About The Insight Partners
14.2 Glossary of Terms
List of Tables
Table 1. US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type – Revenue and Forecast to 2030 (US$ Million)
Table 2. US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 3. US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 4. US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication – Revenue and Forecast to 2030 (US$ Million)
Table 5. US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 6. US: US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 7. Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type – Revenue and Forecast to 2030 (US$ Million)
Table 8. Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 9. Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 10. Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication – Revenue and Forecast to 2030 (US$ Million)
Table 11. Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 12. Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 13. Germany US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type – Revenue and Forecast to 2030 (US$ Million)
Table 14. Germany US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 15. Germany US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 16. Germany US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication – Revenue and Forecast to 2030 (US$ Million)
Table 17. Germany US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 18. Germany US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 19. UK US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type – Revenue and Forecast to 2030 (US$ Million)
Table 20. UK US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 21. UK US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 22. UK US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication – Revenue and Forecast to 2030 (US$ Million)
Table 23. UK US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 24. UK US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 25. France US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type – Revenue and Forecast to 2030 (US$ Million)
Table 26. France US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 27. France US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 28. France US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication – Revenue and Forecast to 2030 (US$ Million)
Table 29. France US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 30. France US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 31. Italy US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type – Revenue and Forecast to 2030 (US$ Million)
Table 32. Italy US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 33. Italy US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 34. Italy US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication – Revenue and Forecast to 2030 (US$ Million)
Table 35. Italy US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 36. Italy US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 37. Spain US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type – Revenue and Forecast to 2030 (US$ Million)
Table 38. Spain US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 39. Spain US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 40. Spain US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication – Revenue and Forecast to 2030 (US$ Million)
Table 41. Spain US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 42. Spain US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 43. Rest of Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by Type – Revenue and Forecast to 2030 (US$ Million)
Table 44. Rest of Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 45. Rest of Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy – Revenue and Forecast to 2030 (US$ Million)
Table 46. Rest of Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by Indication – Revenue and Forecast to 2030 (US$ Million)
Table 47. Italy US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application – Revenue and Forecast to 2030 (US$ Million)
Table 48. Rest of Europe US and Europe Cell and Gene Therapy Manufacturing Services Market, by End User – Revenue and Forecast to 2030 (US$ Million)
Table 49. Recent Organic Growth Strategies in US and Europe Cell and Gene Therapy Manufacturing Services Market
Table 50. Recent Inorganic Growth Strategies in the US and Europe Cell and Gene Therapy Manufacturing Services Market
Table 51. Glossary of Terms
List of Figures
Figure 1. US and Europe Cell and Gene Therapy Manufacturing Services Market Segmentation
Figure 2. US and Europe Cell and Gene Therapy Manufacturing Services Market, by Region
Figure 3. US and Europe Cell and Gene Therapy Manufacturing Services Market Overview
Figure 4. Cell Therapy Segment Held Largest Share of Type Segment in US and Europe Cell and Gene Therapy Manufacturing Services Market
Figure 5. US and Europe Cell and Gene Therapy Manufacturing Services Market, by Country & Region (US$ Million)
Figure 6. US and Europe Cell and Gene Therapy Manufacturing Services Market – Leading Country Markets (US$ Million)
Figure 7. US and Europe Cell and Gene Therapy Manufacturing Services Market – Industry Landscape
Figure 8. US: PEST Analysis
Figure 9. Europe: PEST Analysis
Figure 10. Experts’ Opinion
Figure 11. US and Europe Cell and Gene Therapy Manufacturing Services Market: Key Industry Dynamics
Figure 12. US and Europe Cell and Gene Therapy Manufacturing Services Market: Impact Analysis of Drivers and Restraints
Figure 13. US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue Forecast and Analysis – 2021-2030
Figure 14. Market Positioning of Key Players in US and Europe Cell and Gene Therapy Manufacturing Services Market
Figure 15. US and Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Type 2022 & 2030 (%)
Figure 16. Cell Therapy: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 17. Autologous: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 18. Allogenic: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 19. Gene Therapy: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 20. Viral Vector: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 21. Non-Viral Vector: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 22. US and Europe Cell and Gene Therapy Manufacturing Services Market, by Application 2022 & 2030 (%)
Figure 23. Cancer: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 24. Orthopedics: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 25. Others: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 26. US and Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Application 2022 & 2030 (%)
Figure 27. Clinical Manufacturing: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 28. Commercial Manufacturing: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 29. US and Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by End User 2022 & 2030 (%)
Figure 30. Pharmaceutical & Biotechnology Companies: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 31. Contract Research Organizations (CROs): US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 32. United States: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Key Country – Revenue (2022) (US$ Million)
Figure 33. US: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 34. Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Key Country – Revenue (2022) (US$ Million)
Figure 35. Europe US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 36. Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market, by Country, 2022 & 2030 (%)
Figure 37. Germany: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 38. UK: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 39. France: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 40. Italy: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 41. Spain: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 42. Rest of Europe: US and Europe Cell and Gene Therapy Manufacturing Services Market – Revenue and Forecast to 2030 (US$ Million)
Figure 43. Growth Strategies in US and Europe Cell and Gene Therapy Manufacturing Services Market
The List of Companies - US and Europe Cell and Gene Therapy Manufacturing Services Market
- Thermo Fisher Scientific Inc
- Merck KGaA
- Charles River Laboratories International Inc
- Lonza Group AG
- WuXi AppTec Co Ltd
- Catalent Inc
- Takara Bio Inc
- Nikon Corp
- FUJIFILM Holdings Corp
- National Resilience Inc,
- Oxford BioMedica Plc
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
Trends and growth analysis reports related to US and Europe Cell and Gene Therapy Manufacturing Services Market

Aug 2023
Pediatric Cardiology Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type (Transcatheter Heart Valves, Occlusion Devices, Catheters, Stents, Introducer Sheaths, and Others), Disease Indication (Congenital Heart Disease, Acquired Heart Disease, Arrhythmias, Cardiomyopathies, and Others), Surgical Procedure (Interventional Procedures, Heart Rhythm Management Procedures, and Others), End User (Hospitals, Specialty Clinics, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Aug 2023
Pharmaceutical Membrane Filters Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Technology (Microfiltration, Ultrafiltration, Reverse Osmosis and Nanofiltration), Design (Hollow Fiber, Spiral Wound, Tubular System and Plate and Frame), Material (Polyethersulfone (PES), Polysulfone (PS), Cellulose-Based Membranes, Polytetrafluoroethylene (PTFE), Polyvinyl Chloride (PVC), Polyacrylonitrile (PAN) and Others), End User (Pharmaceutical and Biotech Industries and CROs and CDMOs), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, South & Central America)

Aug 2023
ECG Devices Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Resting ECG and Stress ECG), Lead Type (12-Lead ECG, 3–6 Lead ECG, and Single Lead), Technology [Portable (Wired) ECG System and Wireless ECG System], End User (Hospital and Clinics, Ambulatory Surgical Centers, Cardiac Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Aug 2023
Surgical Laser Fiber Units Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Laser Type (CO2 Laser, Diode Laser, Erbium Laser, Nd:YAG Laser, Holmium Laser, Alexandrite Laser, and Others), Material (Silica-Based Fibers, Quartz Fibers, Polymer-Based Fibers, Multimode Fibers, and Others), Power (Low-Power Lasers, Medium-Power Lasers, and High-Power Lasers), Application (Urology, Dermatology, Gynecology, Cardiology, Neurology, Ophthalmology, Respiratory, Dentistry and Others), Wavelength (9,301 nm and above, 2,941–9,300 nm, and 1,441–2,940 nm, 821–1,440 nm, 710–820 nm, and below 710 nm), End User (Hospitals, Specialty Clinics, Physician Office, and Others), and Geography

Aug 2023
Therapeutic Vaccines Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Cancer Vaccines, Infectious Disease Vaccines, and Others), Technology (Allogenic Vaccines and Autologous Vaccines), End User (Hospitals, Clinics, and Others), and Geography

Aug 2023
Medical Cables Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Disposable Medical Cables, Reusable Medical Cables, and Custom Medical Cables), Applications [Diagnostics (Ultrasound Cables, Endoscopy Cables, Patient Interface Cables, and Others), Motorized Equipment, Patient Monitoring (ECG Cables, SpO2 Cables, NiBP Cables, EEG Cables, and Others), Surgical and Life Support (Fiber Optics, Modular Local Area Network, and Others), and Others], End User (Hospital and Clinics, Diagnostic Laboratories and Imaging Centers, Ambulatory Surgical Centers, and Others), and Geography

Aug 2023
Laser-Assisted ENT Surgeries Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Laser Type (C02 Laser, Nd:YAG Laser, Diode Laser, Blue Laser, KTP Laser, Argon Laser, and Other Laser Types), Surgery Type [Laser Laryngeal Surgery, Laser Endoscopic Sinus Surgery (LESS), Laser-Assisted Uvulopalatoplasty (LAUP), Laser-Assisted Stapedotomy, Laser-Assisted Tonsillectomy and Adenoidectomy, Laser Turbinates Reduction, Transoral Laser Microsurgery (TLM), Nasal Surgery, and Other Surgery Types], End User (Hospitals and Specialty Clinics, Physician Offices, and Other End Users), and Geography (North America, Europe, Asia Pacific, South and Central America, and Middle East and Africa)

Aug 2023
Mobile Cleanroom Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Softwall and Hardwall), End User (Microelectronics Industry, Pharmaceuticals and Biotechnology Industry, Medical Device Manufacturers, and Others), and Geography
 Get Free Sample For
Get Free Sample For