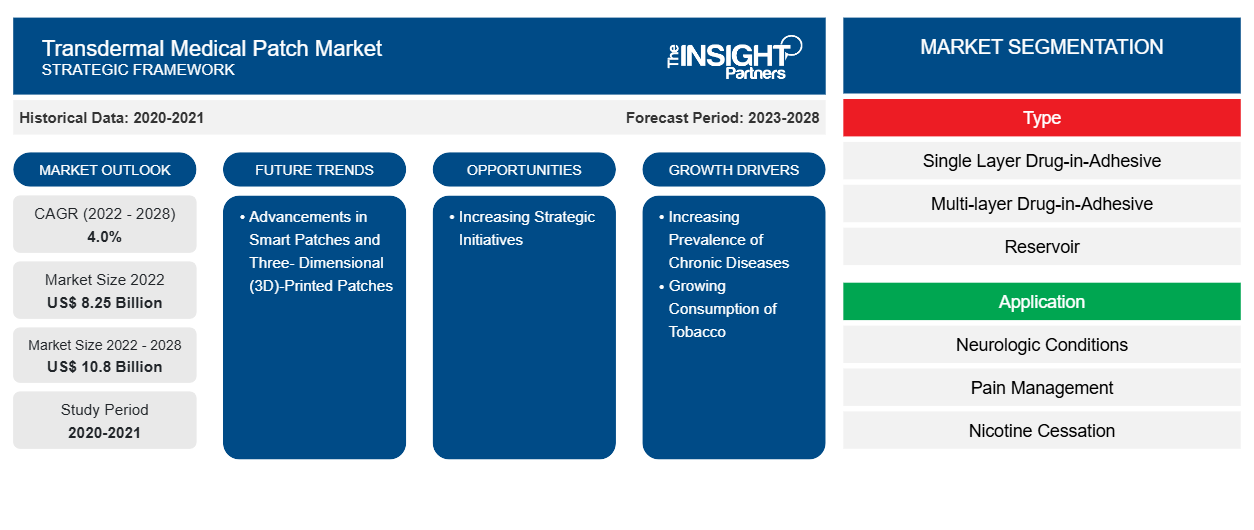
[Research Report] The transdermal medical patch market is projected to reach US$ 10,799.74 million by 2028 from US$ 8,247.21 million in 2022. It is expected to register a CAGR of 4.0% from 2023 to 2028.
Analyst Perspective
The rising prevalence of chronic diseases and growing consumption of tobacco drive the transdermal medical patch market growth. The rising number of patients suffering from chronic conditions and increasing activities in research and development, are the key factors propelling the market for transdermal medical patches. Additionally, increasing activities in research and development, are expected to boost the transdermal medical patch market growth. Furthermore, the transdermal medical patch market key players are focusing on strategic initiatives by launching new products to expand their geographic reach and enhance capacities to cater to a large customer base. For instance, in March 2021, Hisamitsu Pharmaceutical Co., Inc. announced approval for the manufacturing and marketing of ZICTHORU Tapes 75 mg, transdermal, pain treatment NSAID patch for “cancer pain” in Japan. The product is a systemic transdermal formulation developed using Hisamitsu’s TDDS (Transdermal Drug Delivery System) technology. The product's efficacy and safety have been confirmed in clinical trials in patients with cancer pain.
Transdermal Medical Patch Market - Market Overview
A transdermal medical patch, also known as medicated patch or skin patch, is a type of medical device designed to deliver medications or other therapeutic substances through the skin and into the blood stream. These patches are typically thin, adhesive patches that applied directly to the skin allowing the medication to be absorbed gradually over a specified period. Transdermal patches provide a convenient method of medication administration. They can be applied to the skin and worn for a specified duration, often ranging for a few hours to several days, depending on the medication and desired therapeutic effect.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Transdermal Medical Patch Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Transdermal Medical Patch Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Driver
Increasing Prevalence of Chronic Diseases Drives Global Transdermal Medical Patch Market
According to the Centers for Disease Control and Prevention (CDC), chronic diseases such as heart disease, cancer, and diabetes account for a significant amount (US$ 4,100,000 million) in annual healthcare spending in the US. Transdermal medical patches are used to administer medications to people suffering from chronic diseases such as heart disease, stroke, diabetes, cancer, obesity, and arthritis. According to the World Health Organization (WHO), ~17.9 million people died from CVDs in 2019, representing 32% of all deaths recorded globally. 85% of CVD-related deaths were due to heart attacks. According to the same source, over three-quarters of CVD deaths occurred in low-and-middle-income countries in 2019, including 17 million premature deaths (under the age of 70) due to noncommunicable diseases; CVDs were a cause of death in 38% of the mortalities associated with noncommunicable diseases.
A cardiac patch provides an excellent platform for cell engraftment improvement. For example, a vascularized cardiac patch developed recently shows promising potential for treating ischemic heart injuries. Nitroglycerin transdermal patches are used to prevent angina (chest pain) episodes in people with coronary artery disease. It works by relaxing the blood vessels and reducing the stress on the heart, thereby lowering the need for oxygen. However, these patches cannot be used to treat angina attacks that have already begun.
Alzheimer's disease was officially listed as the sixth-leading cause of death in the US in 2019 and the seventh-leading cause of death in 2020 and 2021. According to the 2023 stats by the National Institute of Health, ~6.7 million Americans (i.e., 1 in 9 people or 10.8%), aged 65 and older, have Alzheimer's dementia. The percentage of people with Alzheimer's dementia increases with age. 5% of people aged 65–74, 13.1% aged 75–84, and 33.3% aged 85 and above have Alzheimer's dementia. According to a published the “Global Prevalence of Young-Onset Dementia" report published in 2021, the prevalence of younger-onset dementia in the US is estimated at 110 of every 100,000 people aged 30–64 years, or ~200,000 Americans in total. Thus, individuals with age less than 65 can also develop Alzheimer's dementia.
Rivastigmine patch is used to treat dementia (memory loss) associated with mild, moderate, or severe Alzheimer's disease or mild-to-moderate dementia associated with Parkinson's disease. Rivastigmine and donepezil, which are cholinesterase inhibitors, exhibit a dose–response relationship, with higher doses of the drugs demonstrating greater efficacy. As a small, lipophilic, and hydrophilic molecule, rivastigmine is chemically well-suited for transdermal delivery. The technology underlying the rivastigmine patch contributes to its discreetly small and thin appearance. Thus, the rivastigmine patch enables quick and easy access to high-dose efficacy.
Therefore, the increasing prevalence of chronic diseases catalyzes the demand for medical patches, thus surging transdermal medical patch market.
Transdermal Medical Patch Market - Segmental Analysis
Based on type, the transdermal medical patch market is segmented into single-layer drug in-adhesive, multi-layer drug in-adhesive, reservoir, vapor patch and matrix. The matrix segment held the largest market share in 2022, and the same segment is anticipated to register the highest CAGR of 5.3% during the forecast period. The market position of this segment is due to the patient compliance and convenience. The matrix patch is designed with features that further improve patient compliance, such as extended wear time, simplified application, or smart monitoring capabilities to track medication adherence. The matrix patch incorporates sensors or monitoring capabilities to provide real-time feedback on a patient’s health status.
Transdermal Medical Patch Market - Regional Analysis
Asia-Pacific is the dominating region in the transdermal medical patch market. The Asia-Pacific transdermal medical patch market was valued at US$ 2,024.32 million in 2022 and is projected to reach US$ 2,744.32 million by 2028; it is expected to grow at a CAGR of 5.2% during the forecast period. The Asia-Pacific transdermal medical patch market is segmented into China, the Japan, India, and the Rest of Asia-Pacific. The regional growth is attributed to growing research on cancer and gene mutations, increasing disease modeling, and developments by market players offering transdermal medical patch. Factors such as developing healthcare facilities, growing aging population to share key facts associated with stem cell technologies and increasing partnerships with foreign entities are likely to further propel the transdermal medical patch market growth during the forecast period.
Transdermal Medical Patch Market - Key Player Analysis
The transdermal medical patch market analysis consists of the players such as Hisamitsu Pharmaceutical Co Inc, Medline Industries LP, Johnson & Johnson, Novartis AG, Teva Pharmaceutical Industries Ltd, UCB SA, Viatris Inc, Endo Pharmaceuticals Inc, Boehringer Ingelheim International GmbH, and Corium, LLC. Among the players in the transdermal medical patch market Hisamitsu Pharmaceutical Co Inc and Novartis AG are the top two players owing to the diversified product portfolio offered.
Transdermal Medical Patch Market Regional Insights
Transdermal Medical Patch Market Regional Insights
The regional trends and factors influencing the Transdermal Medical Patch Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Transdermal Medical Patch Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Transdermal Medical Patch Market
Transdermal Medical Patch Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 8.25 Billion |
| Market Size by 2028 | US$ 10.8 Billion |
| Global CAGR (2022 - 2028) | 4.0% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Transdermal Medical Patch Market Players Density: Understanding Its Impact on Business Dynamics
The Transdermal Medical Patch Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Transdermal Medical Patch Market are:
- Hisamitsu Pharmaceutical Co Inc
- Medline Industries LP
- Johnson & Johnson
- Novartis AG
- Teva Pharmaceutical Industries Ltd
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Transdermal Medical Patch Market top key players overview
Transdermal Medical Patch Market - Recent Developments
Inorganic and organic strategies such as mergers and acquisitions are highly adopted by companies in the transdermal medical patch market. A few recent key market developments are listed below:
- In 2019, Nitto Denko Corporation announced the Bisono Tape 2mg, transdermal patches containing a β1 blocker that was co-developed with Toa Eiyo Ltd., launched by Toa Eiyo and Astellas Pharma Inc.
- In June 2022, Hisamitsu Pharmaceutical Co., Inc. has been approved for the additional indications of “low back pain, humeroscapular periarthritis, cervico-omo-brachial syndrome and tenosynovitis” for ZICTHORU Tapes, transdermal, pain treatment NSAID patch in Japan. The product was approved for manufacturing and marketing approval for “analgesia in various cancer” in March 2021. This approval is based on the data of Phase III clinical studies that evaluated the efficacy and safety of the product in patients with low back pain, humeroscapular periarthritis, cervico-omo-brachial syndrome and tenosynovitis. With this approval of the additional indications, Hisamitsu expects the product to be a new option for the treatment of low back pain, humeroscapular periarthritis, cervico-omo-brachial syndrome and tenosynovitis.
- In January 2021, Hisamitsu Pharmaceutical Co., Inc. has commenced the Phase III clinical study in the U.S. for transdermal, pain relief and anti-inflammatory patch for the treatment of Osteoarthritis knee pain. The clinical study will be conducted by the U.S. subsidiary, Noven Pharmaceuticals, Inc. The investigational product is a transdermal formulation developed using Hisamitsu’s TDDS (Transdermal Drug Delivery System) technology. Hisamitsu Pharmaceutical hopes the investigational product will be a new treatment option for Osteoarthritis of the knee, demonstrating efficacy and safety by achieving higher drug delivery to the affected area as one of the transdermal formulation attributes.
- In September 2022, Corium, Inc launched ADLARITY (donepezil transdermal system) and is now available for prescription in the U.S. for treating patients with mild, moderate, or severe dementia of Alzheimer's type.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Small Internal Combustion Engine Market
- Grant Management Software Market
- Industrial Valves Market
- Mobile Phone Insurance Market
- Biopharmaceutical Contract Manufacturing Market
- Online Exam Proctoring Market
- Virtual Production Market
- Battery Testing Equipment Market
- Dealer Management System Market
- Vertical Farming Crops Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Type, Application, and Distribution Channel

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
The matrix segment held the largest share of the market in 2022. Also, the same segment is estimated to register the highest CAGR in the market during the forecast period.
The CAGR value of the transdermal medical patch market during the forecasted period of 2023-2028 is 4.0%.
The factors that are driving the growth of the transdermal medical patch market are the increasing aging population, increasing prevalence of chronic diseases and growing consumption of tobacco and increasing partnerships with foreign entities. These are some of the major factors contributing to the growth of the transdermal medical patch industry.
The Asia Pacific is expected to be the fastest-growing region in the transdermal medical patch market over the forecast period due to the increasing prevalence of chronic diseases and the growing research on cancer and gene mutations, increasing disease modeling, and developments by market players offering transdermal medical patch.
The transdermal medical patch market is expected to be valued at US$ 10,799.74 million in 2028.
The transdermal medical patch market majorly consists of the players, such as Hisamitsu Pharmaceutical Co Inc, Medline Industries LP, Johnson & Johnson, Novartis AG, Teva Pharmaceutical Industries Ltd, UCB SA, Viatris Inc, Endo Pharmaceuticals Inc, Boehringer Ingelheim International GmbH, and Corium, LLC.
The transdermal medical patch is a medicated patch that can deliver drugs through skin portals directly to the bloodstream at a predetermined rate. This is the most comfortable dosage form – it is non-invasive, avoids the gastrointestinal tract and bypasses first-pass metabolism, can have multiday therapy, and can be terminated at any time.
The transdermal medical patch market is estimated to be valued at US$ 8,247.21 million in 2022.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Transdermal Medical Patch Market
- Hisamitsu Pharmaceutical Co Inc
- Medline Industries LP
- Johnson & Johnson
- Novartis AG
- Teva Pharmaceutical Industries Ltd
- UCB SA
- Viatris Inc
- Endo Pharmaceuticals Inc
- Boehringer Ingelheim International GmbH
- Corium, LLC
 Get Free Sample For
Get Free Sample For