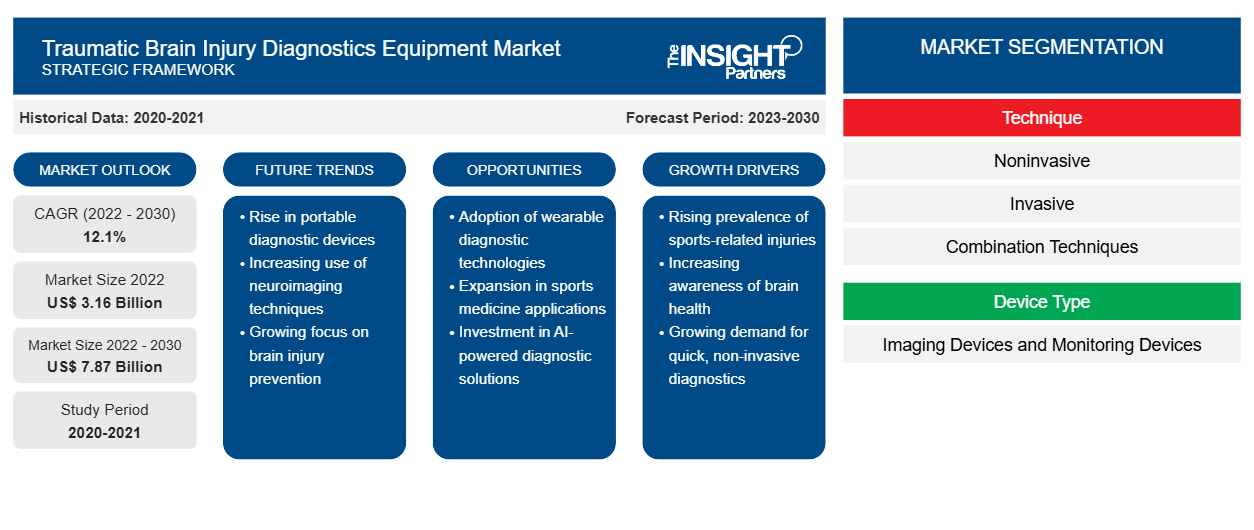
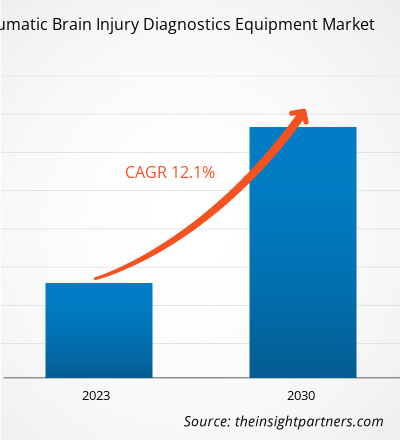
[Research Report] The traumatic brain injury diagnostics equipment market is projected to grow from US$ 3.157 billion in 2022 to US$ 7.872 billion by 2030; the market is estimated to record a CAGR of 12.1% during 2022–2030.
Market Insights and Analyst View:
Factors such as the accelerated demand for advanced diagnostic equipment and quick and effective diagnosis for TBI patients propel the traumatic brain injury diagnostics equipment market growth. However, the adverse effects of contrast medium/agent impede the growth of the market.
Growth Drivers:
Accelerated Demand for Advanced Diagnostic Equipment Drives Traumatic Brain Injury Diagnostics Equipment Market Growth
According to the Medical Research Council 2022 report, 10 million people across the world sustain traumatic brain injury (TBI) annually. Likewise, the Headway 2024 report revealed that acquired brain injury (ABI) is rising in the UK; a total of 356,699 hospital admissions were registered due to ABI in the UK from 2019 to 2020. Among these, the male population was 1.5 times higher than females admitted to hospitals for a head injury. As per The Economist Intelligence Unit report, the global healthcare burden of TBI is estimated to be around US$ 400 billion annually. The most common form of ABI is TBI due to an accident or stroke. The Centers for Disease Control and Prevention (CDC) report revealed that an estimated 1.7 million TBI-related emergency department visits, hospitalizations, and deaths occur annually in the US, especially among adults aged 75 years and older as they are at high risk of falling due to problems with gait and balance. Also, road accidents are the leading cause of TBI-related deaths in the US and are highest among adults aged 20–24 years. Therefore, manufacturers are developing innovative products to diagnose TBI. In October 2023, bioMérieux announced Conformité Européenne (CE) marking for "VIDAS TBI (GFAP, UCH-L1)," a test intended to improve the assessment of patients suffering from mild traumatic brain injury (mTBI). VIDAS TBI (GFAP, UCH-L1) test measures the concentration of glial fibrillary acidic protein (GFAP) and ubiquitin C-terminal hydrolase L1 (UCH-L1)—the two brain biomarkers released into the bloodstream starting from the first hour following a brain injury. It is an easy-to-interpret test providing a test window of up to 12 hours after injury, which can help in shortening total emergency department workup time. The product's commercial launch was in 2023 for selected European, North African, and South American markets; the global launch is planned for 2024 or 2025.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Traumatic Brain Injury Diagnostics Equipment Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Traumatic Brain Injury Diagnostics Equipment Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope:
The “traumatic brain injury diagnostics equipment market analysis” is drawn by considering the following segments: technique, device type, and end user.
Segmental Analysis:
By technique, the traumatic brain injury diagnostics equipment market is segmented into noninvasive, invasive, and combination techniques. The invasive segment held the largest market share in 2022. The non-invasive segment is anticipated to register the highest CAGR of 12.8% during the forecast period.
Noninvasive ultrasound technology is used to efficiently screen the severity of traumatic brain injury (TBI). The EU-funded project "BRAINSAFE" introduced an easy-to-use, handheld, noninvasive absolute intracranial pressure (aICP) meter. This device is an effective alternative to painful, invasive procedures used to assess intracranial injuries. The aICP meter utilizes Doppler ultrasound technology to evaluate blood flow parameters in the ophthalmic artery with the help of a disposable pressure cuff and head frame.
The traumatic brain injury diagnostics equipment market, by device type, is bifurcated into imaging devices and monitoring devices. The imaging devices segment held a larger market share in 2022 and is anticipated to register a considerable CAGR of 11.8% during the forecast period.
The traumatic brain injury diagnostics equipment market, by end user, is categorized into hospitals & clinics, diagnostic centers, and others. The hospitals & clinics segment held the largest market share in 2022 and is anticipated to register a CAGR of 12.0% during the forecast period.
Emergence of Portable Nonivasive Monitoring Devices to Diagnose TBI Patients Acts as a Future Trend in Traumatic Brain Injury Diagnostics Equipment Market
New noninvasive methods for monitoring tissue metabolism can help improve the diagnosis and monitoring of brain conditions such as concussions, stroke, and TBI, owing to which patients can recover more quickly. The consequences of TBI resulted in increasing healthcare burden costs, accounting for US$ 76.5 billion annually, as per the BrainScope company white paper. The installation of "BrainScope One" in less expensive care settings—e.g., emergency department and community settings (including urgent care centers)—may result in a significant reduction of healthcare costs by up to 32.2%, as per the findings revealed in the white paper. BrainScope One aids in eliminating unnecessary CT scans, thereby reducing healthcare costs for TBI.
Further, a team of researchers from the University of Michigan developed a cost-effective, portable, noninvasive tool—Super-Continuum Infrared Spectroscopy of Cytochrome C-Oxidase (SCISCCO) system—to detect neuronal dysfunction. This tool is extremely versatile, having a range of uses from serving as a new device for screening concussion patients to use in the intensive care unit and gauging patients' organ response to treatment.
Likewise, the University of Birmingham 2024 report revealed that researchers from the University of Birmingham have designed and developed the eye-safe device (EyeD)—a novel diagnostic device to detect TBI. It is based on Raman spectroscopy, an optical technique performed simultaneously on fundus imaging and spectroscopic analysis using a smartphone camera. The Raman spectra collected by EyeD from the retina and optic nerves help in analyzing the presence of TBI-specific biochemical changes using the artificial neural network algorithm "SKiNET" as a decision support tool. EyeD is quick, accurate, and noninvasive, causes no additional discomfort, and provides information on the severity of the trauma instantly, owing to which it is highly suitable while using on-site—at the roadside of the unfortunate event or on the sports pitch—to assess TBI.
Regional Analysis:
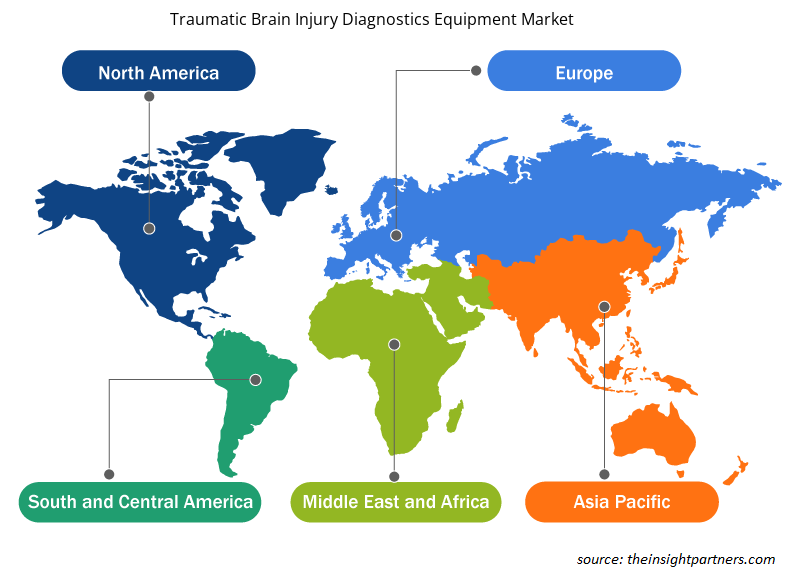
The scope of the global traumatic brain injury diagnostics equipment market report entails North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. In 2022, North America held the largest traumatic brain injury diagnostics equipment market share. The US is the largest contributor to the market in North America. The Centers for Disease Control and Prevention (CDC) report revealed that an estimated 2.5 million people suffer from TBI annually in the US. According to the KNAPP & ROBERTS report, 1 in every 6 Americans live with TBI-related disability in the US alone, accounting for approx. 5.3 million. With the rising prevalence of TBI, the economic cost accounts for US$ 76.5 billion. Among US$ 76.5 billion, US$ 11.5 billion accounts for direct medical costs and nearly US$ 65 billion for indirect costs. The leading causes of TBI include falls (45%), motor vehicle crashes (14.3%), assaults (10.7%), and unknown (19.0%).
Further, companies are launching innovative products for the diagnosis of TBI. For instance, in August 2022, Abbott announced a new blood test for concussions to predict outcomes from brain injury and treatment interventions. The researchers used Abbott's "i-STAT TBI Plasma Test," the only FDA-cleared rapid test intended for concussion, and Abbott's core laboratory ARCHITECT instrument to measure two biomarkers in blood plasma associated with brain injury. The i-STAT TBI Plasma test measures the level of biomarkers released in the bloodstream in response to the brain injury; the level of biomarkers assists in determining the need for a CT scan.
Additionally, Sense Neuro Diagnostics announced clearance to conduct clinical trials for hemorrhage detection. The new trial approved by the FDA Division of Neurosurgical, Neurointerventional, and Neurodiagnostic Devices began in June 2023, including up to 300 patients at 30 US, Canada, and Indian sites. This noninvasive technology has the potential to collect 360 data points within 2.5 seconds to detect brain hemorrhage or stroke type, thereby helping quick response by physicians, emergency department personnels, neuro ICU teams, and military field hospitals assessing and monitoring TBI. Therefore, the traumatic brain injury diagnostics equipment market size is likely to surge due to innovative product launches by US-based companies to improve outcomes of diagnosis of patients suffering from TBI.
Traumatic Brain Injury Diagnostics Equipment Market Regional Insights
Traumatic Brain Injury Diagnostics Equipment Market Regional Insights
The regional trends and factors influencing the Traumatic Brain Injury Diagnostics Equipment Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Traumatic Brain Injury Diagnostics Equipment Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Traumatic Brain Injury Diagnostics Equipment Market
Traumatic Brain Injury Diagnostics Equipment Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 3.16 Billion |
| Market Size by 2030 | US$ 7.87 Billion |
| Global CAGR (2022 - 2030) | 12.1% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Technique
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Traumatic Brain Injury Diagnostics Equipment Market Players Density: Understanding Its Impact on Business Dynamics
The Traumatic Brain Injury Diagnostics Equipment Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Traumatic Brain Injury Diagnostics Equipment Market are:
- GE HealthCare Technologies Inc
- Elekta AB
- Integra LifeSciences Holdings Corp
- Natus Medical Inc
- Raumedic AG
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Traumatic Brain Injury Diagnostics Equipment Market top key players overview
Industry Developments and Future Opportunities:
The traumatic brain injury diagnostics equipment market forecast can help stakeholders in this marketplace plan their growth strategies. A few strategic developments by leading players operating in the market are listed below:
- In October 2023, GE Healthcare Technologies Inc announced a collaboration with Imeka to expand the capabilities of MRI for brain imaging and further develop advanced precision medicine for brain health. Under the collaboration, GE Healthcare Technologies Inc has utilized Imeka’s neuroimaging technology, ANIE biomarker platform, which has broader applicabilities in research and clinical care settings across the spectrum of central nervous system diseases and disorders, such as traumatic brain injury, Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. The ANIE biomarker platform is integrated with artificial intelligence that identifies and quantifies axonal loss, neuroinflammation, and demyelination. Thus, with the collaboration, GE Healthcare Technologies Inc integrated its BrainWave advanced diffusion processing package with the ANIE biomarker platform. The newly integrated technologies have allowed researchers and clinicians to analyze diffusion MRI signals in the brain in greater detail.
Competitive Landscape and Key Companies:
Besides highlighting the factors impacting the market, the traumatic brain injury diagnostics equipment market report showcases the developments of prominent players. GE HealthCare Technologies Inc, Elekta AB, Integra LifeSciences Holdings Corp, Natus Medical Inc, Raumedic AG, BrainScope Co Inc, Luciole Medical AG, Soterix Medical Inc, Medtronic Plc, Vivonics Inc, NanoDx Inc, Compumedics Ltd, Sense Diagnostics Inc, NeuraSignal Inc, and Neurovigil Inc are among the prominent companies operating in the market. These companies focus on developing new technologies, upgrading existing products, and expanding their geographic presence to meet the growing consumer demand worldwide.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Technique, Device Type, End User, and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
The imaging device segment dominated the traumatic brain injury diagnostic equipment and held the largest market share in 2022.
A TBI may be open (penetrating) or closed (non-penetrating). A penetrating TBI occurs when a physical, external forces affect the brain and an object enters the brain tissue. A closed TBI is caused by an external force that produces movement of the brain without skull. Therefore, rising prevalence of TBI increases diagnosis process by the clinicians to assess the severity of TBI.
Key factors that are driving the growth of this market are accelerated demand for advanced diagnostic equipment and quick and effective diagnosis for TBI patients.
The CAGR value of the traumatic brain injury diagnostic equipment during the forecasted period of 2022-2030 is 12.1%.
The traumatic brain injury diagnostic equipment majorly consists of the players such GE HealthCare Technologies Inc, Elekta AB, Integra LifeSciences Holdings Corp, Natus Medical Inc, Raumedic AG, BrainScope Co Inc, Luciole Medical AG, Soterix Medical Inc, Medtronic Plc, Vivonics Inc, NanoDx Inc, Compumedics Ltd, Sense Diagnostics Inc, NeuraSignal Inc, and Neurovigil Inc, and among others.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Traumatic Brain Injury Diagnostic Equipment Market
- GE HealthCare Technologies Inc
- Elekta AB
- Integra LifeSciences Holdings Corp
- Natus Medical Inc
- Raumedic AG
- BrainScope Co Inc
- Luciole Medical AG
- Soterix Medical Inc
- Medtronic Plc
- Vivonics Inc
- NanoDx Inc
- Compumedics Ltd
- Sense Diagnostics Inc
- NeuraSignal Inc
- Neurovigil Inc
 Get Free Sample For
Get Free Sample For