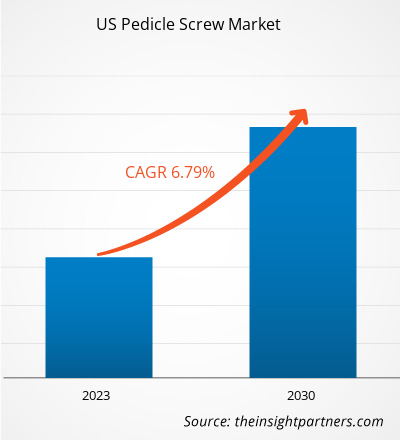
[Research Report] The US pedicle screw market size is expected to grow from US$ 361.28 million in 2022 to US$ 610.85 million by 2030; it is estimated to register a CAGR of 6.79% from 2022 to 2030.
Analyst’s ViewPoint
The US pedicle screw market analysis explains market drivers such as the susceptibility of aging population to spinal disorders and the rising number of spinal surgeries using pedicle screw system. Further, product launches, expansions, collaborations, and partnerships among market players and use of robotics for navigation during screw placement are expected to introduce new trends in the market during 2022–2030. Based on product type, the polyaxial pedicle screw system segment accounted for the largest share in 2022. Based on application, the thoracolumbar fracture segment dominated the market by accounting maximum share. By surgery type segment, the open surgery segment is likely to account for a considerable share of the US pedicle screw market during the forecast period. Based on end user, the hospitals segment is expected to account for a maximum share of the US pedicle screw market during 2022–2030.
Pedicle screws are threaded titanium or stainless-steel implants that are fastened through the vertebral pedicles located at the back of spinal bones. Pedicle screws help secure rods and/or plates to the spinal segment during a spinal fusion surgery. The screw act as an anchor point and are typically positioned at 2 or 3 consecutive spinal segments connected by a short rod.
Market Insights
Susceptibility of Aging Population to Spinal Disorders
The geriatric population is more susceptible to spinal disorders such as degenerative disc diseases, osteoarthritis, scoliosis, spinal tumors, and spinal stenosis. The natural wear and tear of the spine, associated with aging, can lead to several spinal disorders. Moreover, this demographic group poses a unique challenge to spine surgeons due to multiple frailty, comorbidities, decreased nutritional status, and higher probability of postoperative complications. According to the World Health Organization (WHO) data published in October 2022, ~80% of the elderly population would be living in low-and middle-income countries by 2050. Moreover, it is anticipated that the pace of this population aging would be much faster than the pace reported in the past. Pedicle screws are widely used during surgical procedures performed to correct vertebral fracture, vertebral deformity, spinal trauma, spinal tumor, etc. Thus, pedicle screw systems are gaining traction owing to the rising elderly population, which is prone to various spinal disorders. The following figure depicts the cumulative incidence rate (%) among the aging population in two groups, i.e., age under 70 (Group A) and age 70 and older (Group B). Vertebral fracture cases after posterior fusion surgery were higher in Group A than in Group B. Pedicle screws are widely used during surgical procedures performed to correct vertebral fracture, vertebral deformity, spinal trauma, spinal tumor, etc. According to the US Census Bureau 2023 report, the elderly population in the US has grown rapidly in the last few decades. During 2010–2020, the population aged 65 and above saw the largest and fastest growth since the decade of 1880–1890; this population reached 55.8 million or 16.8% of the total population in 2020. This upsurge is primarily attributed to the aging of baby boomers, born between 1946 and 1964.
Fig 1: Number of Patients at Risk of Vertebral Fractures
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Source: Nakahashi et al. BMC Musculoskeletal Disorders,2019
Postoperative vertebral fractures with pedicle screw fixation are relatively common complications among ageing population. The surgical management of vertebral fractures and spinal disorders often requires pedicle screw fixation. For instance, surgical management of scoliosis includes the decompression of neutral elements and fusion to realign the spinal segment pedicle screw system.
Future Trend
Use of Robotics for Navigation During Screw Placement
In recent years, the pedicle screw market has witnessed the advent of robotic navigation, especially in minimally invasive spinal surgeries (MISS) has led to an improvement in the accuracy of pedicle screw placement, as robotics aid in screw trajectory planning, size, and real-time visualization during the surgery. In surgeries related to lumbar interbody fusion, the applications of robotics have reached beyond pedicle screw placement. According to an article published in the Journal of Bone and Joint Surgery in 2020, robotic devices facilitate the placement of pedicle screws in patients with difficult anatomy, resulting in increased procedure accuracy, feasibility, and efficiency. The US FDA has approved the use of robotics to analyze the ideal trajectory for pedicle screw placement. The robotic devices serve as a semiactive surgical assistive tool that allows surgeons to broaden their ability to treat patients.
- In 2022, Beijing TINAVI Medical Technologies introduced TiRobot, an orthopedic robot developed as a new technology to aid in minimally invasive, percutaneous pedicle screw placement. This technique is accurate and safe, potentially reducing intraoperative blood loss, shortening operative time, improving fixation accuracy, and reducing surgical risk in the aging population.
- In May 2021, CUREXO Corp., a medical robot specialist company, received a US FDA license for developing the CUVIS-Spine spinal surgery robot. It is a next-generation spinal surgery robot that guides the insertion of a pedicle screw and functions according to the surgery plan by using a high-precision robot arm, wireless one-step navigation based on a real-time OTS sensor to confer precise, safe, and faster operations. CUVIS-Spine also minimizes the filming and reduces the radiation exposure of patients and medical staff.
- In May 2022, Altus Spine, a pioneer in the development and innovation of medical devices used in spinal correction surgeries, announced FDA 510 (K) clearance for Monaco HA Pedicle Screw System. This system comprises a low-profile construct and insertion devices, featuring an optimized surface intended for enhanced fixation. This surfaced is made of calcium and phosphorous to enhance the fixation of a pedicle screw into the surrounding bone.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
US Pedicle Screw Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
US Pedicle Screw Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Product Type-Based Insights
Based on product type, the US pedicle screw market is segmented into polyaxial pedicle screw system, monoaxial pedicle screw system, and other pedicle screw systems. The polyaxial pedicle screw system segment held the largest market share in 2022 and is expected to record a significant CAGR during 2022–2030. Polyaxial pedicle screw systems are used in spinal surgeries designed to stabilize and immobilize the spine. Screws are anchored in the pedicle, which are small bony projections on the vertebral bodies. This type of screw can move in multiple directions, giving surgeons greater flexibility during pedicle placement. In recent years, minimally invasive surgery has been a preferred approach for spinal surgeries using posterior pedicle screw fixation.
Companies Offering Polyaxial Pedicle Screw Systems
Companies | Polyaxial Pedicle Screw System |
Auxien Medical | VERTAUX – Polyaxial Pedicle Screw with Cap |
Stryker | Xia3 |
B. Braun | S4 Spinal System (Monoaxial and Polyaxial) |
Zimmer Biomet | Vital Spinal Fixation System |
Source: Company Websites and The Insight Partners Analysis
The increasing number of spinal surgeries performed and technological advancements in polyaxial pedicle screw systems coupled with rising number of minimally invasive surgeries is expected to bolster the growth of the market for this product type.
Application-Based Insights
Based on application, the global US pedicle screw market is segmented into thoracolumbar fracture, spinal tumor, failed spinal fusion, scoliosis, and spondylitis. The thoracolumbar fracture segment held the largest market share in 2022 and is anticipated to register the highest CAGR during 2022–2030. Thoracolumbar fractures can significantly affect the quality of life, resulting in neurological deficits, deformities, and pain. According to an article published in Orthopaedic Surgery in 2020, spinal fractures account for 32.8/100,000 per population, and thoracolumbar fractures account for ~90% of spinal fractures. In addition, nearly 20% of these fractures are burst fractures due to axial pressure. Most of the thoracolumbar fractures occur between T11 and L2, a stress concentration area.
Pedicle screws are mostly used to correct thoracolumbar burst fractures and are considered the gold standard for spinal internal fixation in posterior surgery. The commonly used pedicle screw systems comprise 4 nails and 2 rods. A large number of biochemical studies have been carried out using pedicle screws. Although pedicle screw fixation has been proven advantageous in correcting spinal fractures, the disadvantage of screw loosening persists in patients with osteoporosis, leading to the failure of the correction procedure.
Surgery Type-Based Insights
In terms of surgery type, the US pedicle screw market is bifurcated into open surgery and minimal invasive surgery. The open surgery segment held a larger share of the market in 2022 and the minimally invasive segment is anticipated to register a higher CAGR during 2022–2030.
Minimally invasive surgeries (MIS) have gained traction owing to the rising application of technologically advanced robotic and image-guided (IG) surgeries. The market for the MIS segment is anticipated to grow six times faster as compared to the open surgeries segment during the forecast period. These surgeries are associated with less damage to the surrounding muscles, lower bleeding, minimum pain, faster recovery, and shorter hospital stays. At present, MIS is considered a common procedure for spinal fusion.
Key US pedicle screw market players are focusing on expanding their portfolios of product types supporting minimally invasive surgeries. In September 2021, Alphatec Holdings, Inc. launched the InVictus OsseoScrew spinal fixation system, which is an expandable pedicle screw system. Thus, with the growing preference for MIS among surgeons and patients, and the burgeoning prevalence of spinal cord degenerating diseases, the US pedicle screw market is set to pick pace during the forecast period.
End User-Based Insights
By end user, the market is segregated into hospitals, ambulatory surgical centers, and specialty clinics. The hospital segment held the largest share of the market in 2022 and is anticipated to register the highest CAGR in the US pedicle screw market during 2022–2030.
US Pedicle Screw Market, by Product Type – 2022 and 2030

- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Country Analysis
The growth of the US pedicle screw in the US is attributed due to unprecedented shift toward aging population prone to spinal disorders and rising number of spinal surgeries using pedicle screw systems. However, regulatory hurdles and challenges associated with pedicle screw fixation hinder the market growth.
Major market players operating in the US pedicle screw include DePuy Synthes, Stryker, and Intelivation Technologies. These companies engage in product type developments and launches to remain competitive in the market. In the US, technologically advanced pedicle screws are widely adopted for various minimally invasive and open surgeries.
Following is the list of major developments undertaken by the companies in the pedicle screw ecosystem:
- In May 2023, Intelivation Technologies launched a new minimally invasive pedicle screw system. The Golden Isles is a percutaneous system that improves procedural flexibility and visualization for surgeons, resulting in minimal soft tissue disruption in patients.
- In March 2022, SurGenTec, a US-based privately held company specializing in spine and orthopedic technology, received US FDA approval for its standalone spine fixation implant—the ION Screw.
- In February 2022, OsteoCentric Spine, LLC, announced its plans to enter the pedicle screw fixation market after executing a private-label deal with Altus Spine. The arrangement allows OsteoCentric to integrate an existing FDA-cleared system with its UnifiMI mechanical integration technology platform.
- In October 2020, Stryker announced that its Mesa Pedicle Screw (launched in 2006) reached the count of 1 million implantations globally. This system was featured during the North American Spine Society (NASS) annual meeting in 2020.
An increase in the number of cases of lumbar fracture, spinal deformities, and spinal tumors in the US population and technological advancements in pedicle screws favor the pedicle screw market. Lumbar fracture is the most common spinal fracture, which includes a complex injury and a high disability rate and causes serious psychological and economic stress in patients.
The report profiles leading players operating in the global US pedicle screw market. These include DePuy Synthes Inc, Zimmer Biomet Holdings Inc, Globus Medical Inc, B. Braun SE, Stryker Corp, Medtronic Plc, Surgalign Holdings Inc, NuVasive Inc, Orthofix Medical Inc, and Alphatec Holdings Inc.
- In August 2022, Surgalign Holdings, Inc. received FDA 510(k) approval of the CorteraTM Spinal Fixation System. This new flagship product from Surgalign is a key piece to the foundational portfolio designed to drive the company’s future growth over the next 10 years.
- In September 2021, Alphatec Holdings, Inc., a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, launched of the InVictus OsseoScrew Expandable Spinal Fixation System.
Company Profiles
- DePuy Synthes Inc
- Zimmer Biomet Holdings Inc
- Globus Medical Inc
- B. Braun SE
- Stryker Corp
- Medtronic Plc
- Surgalign Holdings Inc
- NuVasive Inc
- Orthofix Medical Inc
- Alphatec Holdings Inc
US Pedicle Screw Market Regional Insights
The regional trends and factors influencing the US Pedicle Screw Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses US Pedicle Screw Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for US Pedicle Screw Market
US Pedicle Screw Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 361.28 Million |
| Market Size by 2030 | US$ 610.85 Million |
| Global CAGR (2022 - 2030) | 6.79% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2030 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered | United State
|
| Market leaders and key company profiles |
US Pedicle Screw Market Players Density: Understanding Its Impact on Business Dynamics
The US Pedicle Screw Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the US Pedicle Screw Market are:
- DePuy Synthes Inc
- Zimmer Biomet Holdings Inc
- Globus Medical Inc
- Hill-Rom Holdings Inc
- Stryker Corp
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the US Pedicle Screw Market top key players overview
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product Type, Application, Surgery Type, End User, and Country Analysis

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
The key factor that is driving the pedicle screws market growth include increased adoption of these devices in treating degenerative spinal disorders, and the rising number of life-saving surgeries.
Pedicle screws are used sometimes in a spinal fusion to add extra support and strength to the fusion while it heals. Pedicle screws are placed above and below the vertebrae that were fused. A rod is used to connect the screws which prevents movement and allows the bone graft to heal. After the fusion is completely healed, the screws and rods can be removed. Removal isn't necessary unless they cause the patient discomfort.
The pedicle screws market, by product type, is fragmented into polyaxial pedicle screw system, monoaxial pedicle screw system, and other pedicle screw systems. The polyaxial pedicle screw system segment held the largest market share in 2022 and services segment is anticipated to register the highest CAGR during the forecast period.
The US pedicle screws market, by end user, is fragmented into hospitals, ambulatory surgical centers, and specialty clinics. The hospitals segment held the largest market share in 2022 and ambulatory surgical center anticipated to register the highest CAGR during the forecast period.
US pedicle screws market comprises top players such as Johnson & Johnson (Depuy Synthes), Zimmer Biomet, Globus Medical, Inc., B. Braun Melsungen AG, Orthofix International N.V., Stryker, Medtronic PLC, RTI Surgical, Inc and NuVasive.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - US Pedicle Screw Market
- DePuy Synthes Inc
- Zimmer Biomet Holdings Inc
- Globus Medical Inc
- Hill-Rom Holdings Inc
- Stryker Corp
- B. Braun SE
- Medtronic Plc
- Surgalign Holdings Inc.
- NuVasive Inc
- Orthofix Medical Inc
- Alphatec Holdings Inc
 Get Free Sample For
Get Free Sample For