In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Share 2031
In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market: Size and Share
-
CAGR (2021 - 2028)12.7% -
Market Size 2021
US$ 2.96 Billion -
Market Size 2028
US$ 6.83 Billion

Market Dynamics
- Rising need for faster drug approval
- Increased demand for cost-effective trials
- Advancements in simulation technologies
- Use of digital twins in drug testing
- Increased regulatory support for simulations
- Integration with patient-specific data
- Expansion in personalized medicine research
- Growth in AI-driven predictive models
- Demand in complex disease modeling
Market Segmentation
 Organization Size
Organization Size - Small & Medium Organizations
- Large Organizations
 Offerings
Offerings - Products
- Platforms
- Services
 Application
Application - Product Design & Discovery
- Product Development
- Pre-Clinical Targeting
- Assessment of Drugs & Other Biomedical Products
 Clinical Indication
Clinical Indication - Cardiovascular Diseases
- Neurodegenerative Diseases
- Oncology
- Rare Diseases
- Metabolic Diseases
- Immune Based Diseases
- Infectious Diseases
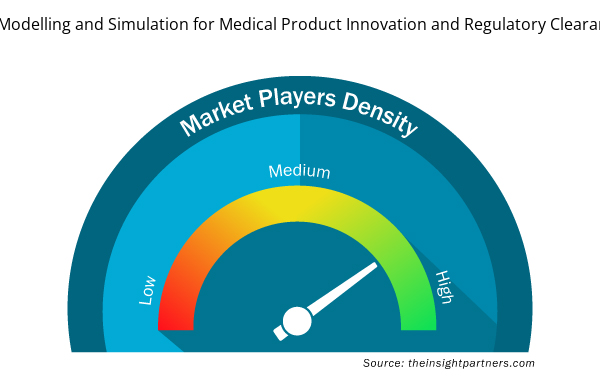
In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market Players Density: Understanding Its Impact on Business Dynamics
The In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market are:
- InSilico Trials Technologies
- Feops
- CADFEM Medical GmbH
- Dassault Systèmes
- Virtonomy GmbH
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the In Silico Trials: Computational Modelling and Simulation for Medical Product Innovation and Regulatory Clearance Market top key players overview
 Get Free Sample For
Get Free Sample For