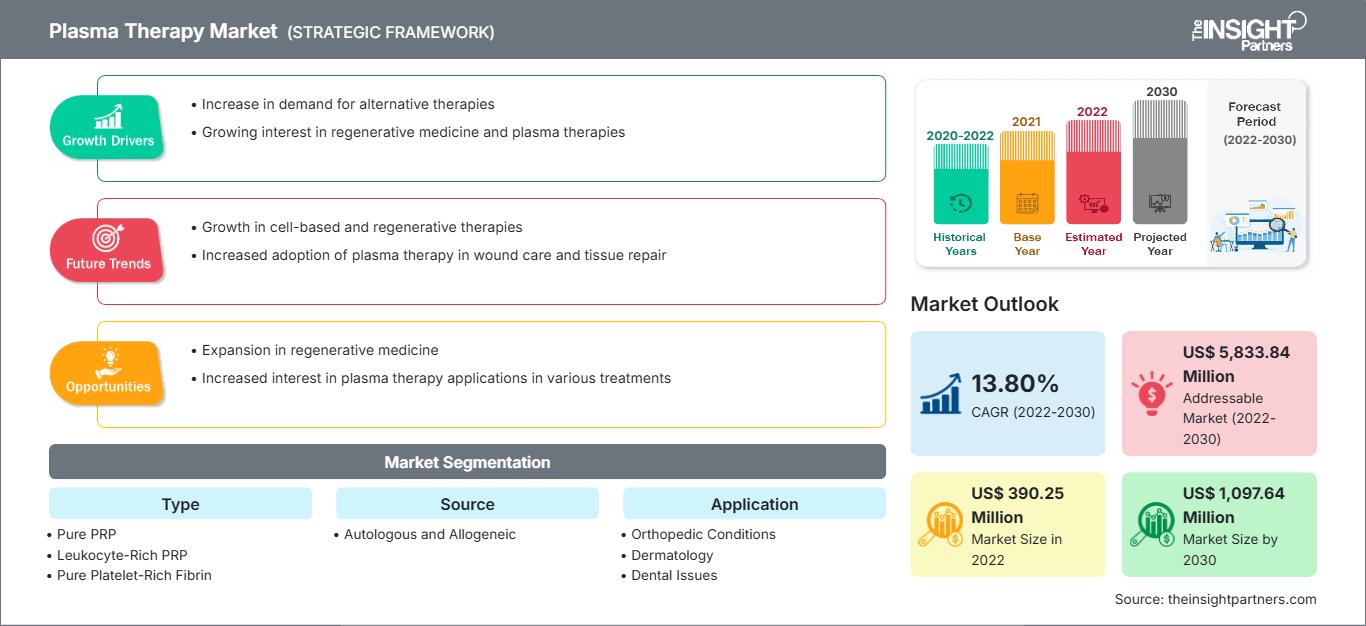
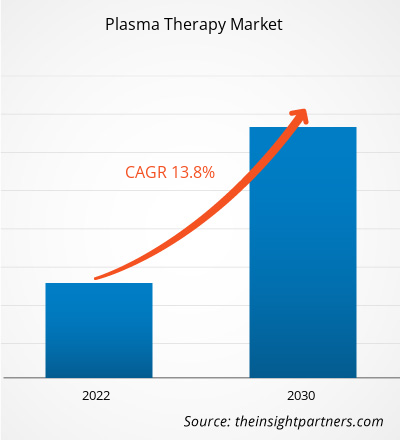
[Research Report] The plasma therapy market size is expected to grow from US$ 390.25 million in 2022 to US$ 1,097.64 million by 2030; the market is estimated to register a CAGR of 13.80% from 2022 to 2030.
Analyst’s Viewpoint
The plasma therapy market analysis explains growth drivers such as the rising cases of orthopedic disorders in people, especially in the geriatric population; rising number of joint and muscle injuries in athletes, and surging demand for minimally invasive cosmetic procedures. Further, the customization of plasma therapies according to patients’ needs is expected to introduce new trends in the market during 2022–2030.
Plasma therapy, also termed as platelet-rich plasma (PRP) therapy, entails the utilization of the blood plasma obtained from individuals who have successfully recovered from an illness to address the range of medical conditions and foster the process of healing. PRP treatments find applications in orthopedics for the treatment of joint injuries, dermatology in skin rejuvenation procedures, and dentistry for tissue repair. The growth factors present in PRP effectively stimulate the regeneration of tissues, thereby expediting the healing of injuries or enhancing mechanisms involved in aesthetic procedures.
Market Insights
Rising Cases of Orthopedic Disorders & Muscle Injuries and Increasing Use of PRP in Aesthetic Procedures Fuel Plasma Therapy Market
According to statistics published by the World Health Organization (WHO) in 2023, ~528 million people across the world were living with osteoarthritis (OA) in 2019, representing an increase of 113% since 1990. An article published in PubMed Central in December 2020 states that the use of PRP offers potential for tissue repair due to the abundance of growth factors and cytokines in this plasma, which are crucial in initiating and modulating regenerative microenvironments for both soft and hard tissues. PRP therapy can be used in the healing of bone fractures; ligament, muscle, and tendon injuries; peripheral nerve injuries; articular cartilage lesions; and osteoarthritis. Therefore, there is a high demand for plasma therapies in orthopedic and tissue repair procedures.
Moreover, PRP is used in cosmetic surgeries for skin rejuvenation, anti-aging, and other aesthetic procedures. Out of 73 studies evaluated in a review published in PubMed Central in November 2021, 45 studies focused on PRP monotherapy, while 35 studies investigated the effects of PRP in combination with micro needling. As a result, the therapies were well-tolerated and suitable for all phototypes. Positive treatment outcomes were identified for various conditions, including skin rejuvenation, scar revision, alopecia, pigmentary disorders, lichen scleroses, leprosy-induced peripheral neuropathy, plaque psoriasis, and nail disorders.
Therefore, the use of PRP in cosmetic surgeries contributes to the growth of the plasma therapy market.
Future Trend
Customization Options to Introduce Patient-Centric Therapies Emerge as Future Trend in Plasma Therapy Market
Customized plasma therapy entails personalizing plasma therapies to meet patients’ needs based on factors such as their specific medical conditions and genetic makeup. Customization efforts are focused on improving the efficacy of plasma therapy by tailoring the composition of components such as antibodies and proteins to treat the specific health condition of the patient more effectively. Researchers and healthcare professionals are implementing their expertise to come up with ways to tailor plasma therapy to specific ailments such as autoimmune disorders, infectious diseases, and rare cancer types. Companies such as Regenexx and Bridging Biosciences provide highly pure and concentrated PRP customizable to eliminate inconsistencies for healing of tissue damage and cell regeneration. USA Medical Research Institute in Texas offers PRP TruDose therapy, which contains personalized therapeutic doses for the treatment of various chronic pain for musculoskeletal conditions, LUPUS, food sensitivities, Lyme disease, and many degenerative conditions.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Plasma Therapy Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope
Type-Based Insights
Based on type, the plasma therapy market is segmented into pure PRP, leukocyte-rich PRP, pure platelet-rich fibrin, and leukocyte-rich fibrin. The pure platelet-rich fibrin segment held the largest market share in 2022 and is anticipated to register the highest CAGR during 2022–2030. Leukocyte-rich PRP (LR-PRP) is a type of PRP that has a higher concentration of neutrophils (white blood cells) than the baseline level. It is generally associated with proinflammatory effects. However, in some cases, LR-PRP can be beneficial to stimulate inflammation in the body to indicate a chronic condition such as tendinopathy.
Source-based Insights
The plasma therapy market, based on source, is bifurcated into autologous and allogeneic. The autologous segment dominated the market in 2020.
Application-based Insights
The plasma therapy market, by application, is segmented into orthopedic conditions, dermatology, dental issues, and others. The dermatology segment held the largest market share in 2022.
End User-Based Insights
In terms of end user, the plasma therapy market is categorized as hospitals and clinics, research institutes, ambulatory surgical centers (ASCs), and others. The hospital segment held the largest share of the market in 2022 and is anticipated to register the highest CAGR in the market during 2022–2030.
Plasma Therapy Market Regional InsightsThe regional trends and factors influencing the Plasma Therapy Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Plasma Therapy Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Plasma Therapy Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 390.25 Million |
| Market Size by 2030 | US$ 1,097.64 Million |
| Global CAGR (2022 - 2030) | 13.80% |
| Historical Data | 2020-2022 |
| Forecast period | 2022-2030 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Plasma Therapy Market Players Density: Understanding Its Impact on Business Dynamics
The Plasma Therapy Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Plasma Therapy Market top key players overview
Regional Analysis
North America dominated the plasma therapy market in 2022. It is further expected to record the highest CAGR in the market during 2022–2030. The market in North America is segmented into the US, Canada, and Mexico. The US holds the largest market share of the global plasma therapy market. Market growth in the country is attributed to the burgeoning adoption of platelet-rich plasma (PRP) in orthopedic and cosmetic surgeries, rising investments in the healthcare sector, dynamics research and development landscape, and USFDA approval for selected PRP therapies.
The report profiles leading players operating in the global plasma therapy market. These include BioLife Plasma Services; Takeda Pharmaceutical Company Limited; Biotest AG CSL Ltd.; Grifols, S.A.; Kedrion S.p.A; ImmunoTek Bio Centers; Bio Products Laboratory Ltd.; China Biologic Products Holdings, Inc.; Octapharma AG; and Origin, Inc. These leading players focus on expanding and diversifying their market presence and clientele, thereby tapping prevailing business opportunities.
Key Developments by Major Market Players are:
- In March 2023, BioLife Plasma Services announced the opening of its plasma donation center in the US with new locations in West Springfield (Massachusetts) and Pearland (Texas). This expansion would help the company expand its business network.
- In February 2021, Atlas Health Medical Group launched the SkinPen. This small pen-shaped microneedling device containing PRP for facelifts punctures the skin and stimulates an anti-aging response.
- In March 2020, Takeda Pharmaceutical Company Limited initiated the development of an anti-SARS-CoV-2 polyclonal hyperimmune globulin (H-Ig) to treat individuals suffering from severe COVID-19.
Frequently Asked Questions
Who are the key players in the plasma therapy market?
Which region is expected to witness significant demand for plasma therapy market in the coming years?
What is the market CAGR value of plasma therapy market during forecast period?
What is plasma therapy?
Which application held the largest share in the plasma therapy market?
What are the driving factors for the plasma therapy market across the globe?
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





















 Get Free Sample For
Get Free Sample For