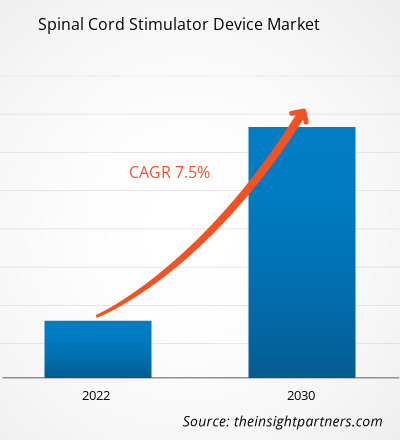
[Research Report] The spinal cord stimulator device market is expected to grow from US$ 2,779.91 million in 2022 to US$ 4,967.52 million by 2030; it is anticipated to record a CAGR of 7.5% from 2022 to 2030.
Market Insights and Analyst View:
A spinal cord stimulation device is an implanted device that relieves pain by transmitting low-level electrical signals directly into the spinal cord. The stimulator is mostly used after nonsurgical pain treatments fail to provide sufficient relief. Additionally, the device can improve the overall quality of life and sleep. As it is typically prescribed along with other pain management treatments, it effectively suppresses the need for pain medicines. The spinal cord stimulator device market size is expanding with the rising number of spinal cord injuries (SCIs) and the availability of innovative treatments.
Growth Drivers and Challenges:
According to the National Library of Medicine (NLM), nearly 250,000–500,000 patients suffer from spinal cord injuries (SCIs) every year worldwide. According to a report published by the Association for Spinal Injury Research, Rehabilitation and Reintegration (Aspire, UK), ~2,500 people are diagnosed with SCIs annually in the UK. Currently, ~50,000 people are living with these injuries in the UK. The National Spinal Cord Injury Statistical Center (NSCISC) estimates that ~320,000 people are living with traumatic SCIs in the US. An elevated number of cases is attributed to motocross and motorcycling competitions emerging as the most popular sporting activities worldwide, as per the National Institute of Health (NIH) report.
Moreover, these injuries are common in the elderly population. According to the data presented under the World Bank's collection of development indicators in 2022, 22.41% of the population in Germany is aged above 65 years. An article published by MDPI mentions that the prevalence rate of traumatic SCIs is ~500 per million inhabitants, and the incidence rate surges to 13 per million globally.
SCIs have life-changing impacts on the injured patients and their families. The rising incidence of SCIs and the corresponding treatment of patients in hospitals is followed by at least one readmission of the patient every year. This is because SCIs are not treated at once. The causes of rehospitalization include respiratory and urinary tract infections and fractures occurring during motorcycling sports activities. With rising SCI and the availability of well-developed infrastructure in treatment centers providing rehabilitation programs, demand for spinal cord stimulation devices is surging in specialized or rehab centers, fueling the growth of the spinal cord stimulator device.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Spinal Cord Stimulator Device Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Report Segmentation and Scope:
The spinal cord stimulator device market is divided based on product type, application, end user, and geography. Based on product type, the spinal cord stimulator device market is bifurcated into rechargeable and non-rechargeable. The spinal cord stimulator device market is segmented based on application into failed back syndrome, complex regional pain syndrome, degenerative disk disease, and others. In terms of end user, the market is categorized into hospitals, ambulatory surgery centers, and others. Based on geography, the spinal cord stimulator device market is segmented into North America (US, Canada, and Mexico), Europe (UK, Germany, France, Italy, Spain, Russia, and Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Australia, Southeast Asia, and Rest of Asia Pacific), the Middle East & Africa (UAE, Saudi Arabia, South Africa, and Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Segmental Analysis:
The spinal cord stimulator device market is segmented based on application into failed back syndrome, complex regional pain syndrome, degenerative disk disease, and others. The complex regional pain syndrome segment held the largest market share in 2022. It is expected to register the highest CAGR during 2022–2030. Complex regional pain syndrome (CRPS) involves prolonged pain and inflammation occurring due to an injury or other medical events such as surgery, trauma, stroke, and heart attack. CRPS can occur anywhere in the body, usually affecting the arm, leg, hand, or foot. It is more likely to occur in anyone at any age, with the inflammation approaching a peak after 40 years. Most CRPS cases are caused by damaged or improperly functioning small peripheral C-fiber nerve fibers, which carry pain signals to the brain. CRPS often remains undiagnosed with the development of tiny clots, sometimes blocking blood flow to nerves and injuring them.
Spinal Cord Stimulator Device Market, by Application – 2022 and 2030
Regional Analysis:
Based on geography, the spinal cord stimulator device market is divided into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America. North America is the largest contributor to the growth of the global spinal cord stimulator device market. Asia Pacific is expected to record the highest CAGR in the spinal cord stimulator device market from 2022 to 2030. The continuously rising incidence of spinal cord injuries is associated with the growth of this market. Currently, spinal cord injuries pose a major health burden in North American countries. According to Spinal Cord Inc., ~18,000 people in the US suffer from these injuries every year.
Spinal Cord Stimulator Device Market Regional InsightsThe regional trends and factors influencing the Spinal Cord Stimulator Device Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Spinal Cord Stimulator Device Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Spinal Cord Stimulator Device Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 2,779.91 Million |
| Market Size by 2030 | US$ 4,967.52 Million |
| Global CAGR (2022 - 2030) | 7.5% |
| Historical Data | 2020-2022 |
| Forecast period | 2022-2030 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Spinal Cord Stimulator Device Market Players Density: Understanding Its Impact on Business Dynamics
The Spinal Cord Stimulator Device Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Spinal Cord Stimulator Device Market top key players overview
Industry Developments and Future Opportunities:
Key initiatives by major players operating in the Spinal Cord Stimulator Device Market are listed below:
- In August 2022, Abbott Laboratories received approval from the US FDA for its Proclaim Plus SCS system to treat chronic pain. The BurstDR stimulation technology enables the device to deliver superior pain relief. It offers FlexBurst360 therapy that offers pain coverage across the trunk and limbs; the device can be programmed per the patient's evolving therapeutic needs.
- In December 2022, the Eterna spinal cord stimulation (SCS) system from Abbott Laboratories will be available to treat chronic pain. Abbott's low-dose BurstDR stimulation, a patented low-dose SCS waveform technology with the greatest clinical evidence, has been approved by the FDA. Additionally, it has been demonstrated that the technique aids in 23% more pain reduction than the results produced by conventional waveform technology-based methods.
Competitive Landscape and Key Companies:
A few of the prominent players operating in the spinal cord stimulator device market are Boston Scientific Corp; Nevro Corp; Abbott Laboratories; Cirtec Medical Corp; Medtronic Plc; Biotronik SE & Co KG; Curonix LLC; Nalu Medical, Inc.; and Synapse Biomedical Inc. These companies focus on new product launches and geographic expansions to meet global consumer demand and increase their product range in specialty portfolios. Their global presence allows them to serve a large base of customers, subsequently facilitating market expansion.
Frequently Asked Questions
Which segment is dominating the spinal cord stimulator device market?
Which region is dominating the spinal cord stimulator device market?
Who are the major players in the spinal cord stimulator device market?
What are the driving and restraining factors for the spinal cord stimulator device market?
What is spinal cord stimulator device?
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





















 Get Free Sample For
Get Free Sample For