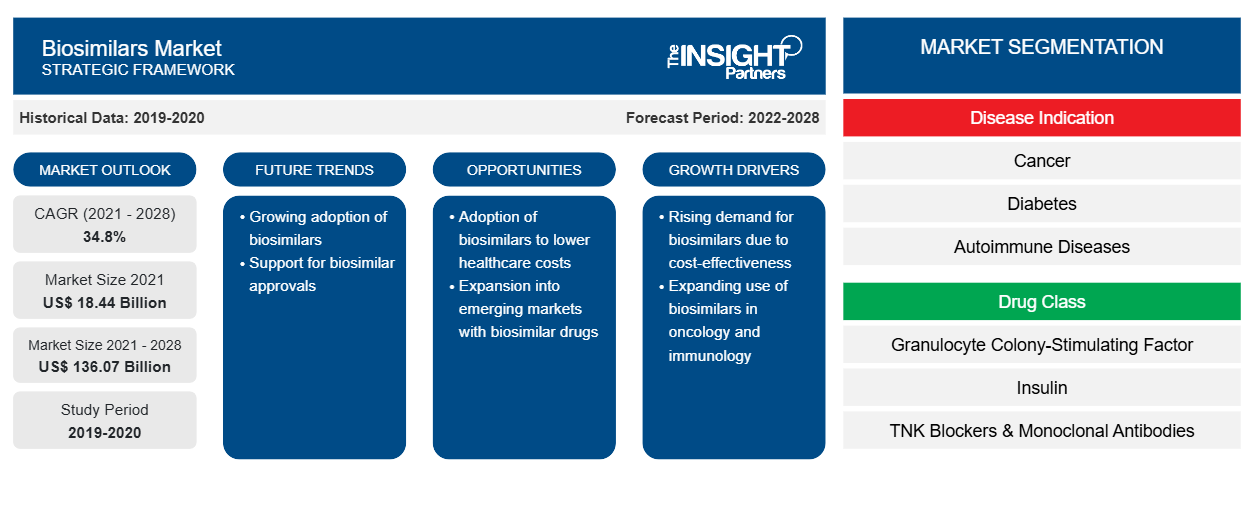
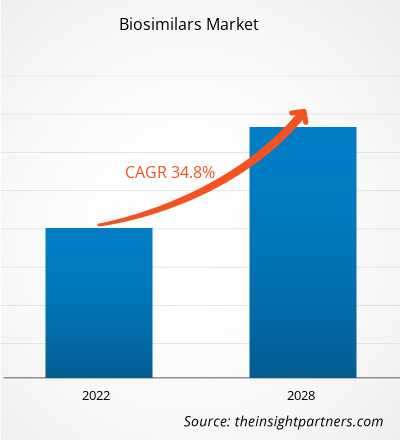
[研究报告] 生物仿制药市场规模预计将从 2021 年的 184.3589 亿美元增长至 2028 年的 1360.6953 亿美元;预计 2022 年至 2028 年的复合年增长率为 34.8%。
分析师观点
推动生物仿制药市场增长的主要因素是癌症等慢性病发病率的不断上升。癌症负担加重以及由此导致的死亡人数不断增加,产生了对负担得起的治疗的需求,从而促进了生物仿制药市场的增长。主要市场参与者还通过各种战略活动(如产品发布、并购)预测期内市场增长。强直性脊柱炎和类风湿性关节炎等自身免疫性疾病的患病率增加推动了生物仿制药市场规模的增长。例如,根据 2020 年《斯堪的纳维亚风湿病学杂志》上发表的一篇题为“西班牙强直性脊柱炎患病率”的论文,约 7.3% 的人口对强直性脊柱炎的筛查呈阳性。生物仿制药,例如英夫利昔单抗-axxq(Avsola)、英夫利昔单抗-qbtx(Ixifi)、英夫利昔单抗-dyyb(Inflectra)和英夫利昔单抗-abda(Renflexis)用于治疗关节炎慢性疼痛。
市场概况
生物仿制药是一种与另一种已获批准的生物药物(“参考药物”)高度相似的生物药物。生物仿制药的批准遵循适用于所有生物药物的相同药品质量、安全性和有效性标准。生物仿制药是许多疾病的安全有效治疗选择,例如慢性皮肤病和肠道疾病(如牛皮癣、肠易激综合征、克罗恩病和结肠炎)、关节炎、肾脏疾病和癌症。生物仿制药可以以较低的成本增加获得救命药物的机会。生物仿制药市场的主要驱动力是慢性病发病率的增加。
定制此报告以满足您的需求
您可以免费定制任何报告,包括本报告的部分内容、国家级分析、Excel 数据包,以及为初创企业和大学提供优惠和折扣
- 获取此报告的关键市场趋势。这个免费样品将包括数据分析,从市场趋势到估计和预测。
市场驱动因素
生物仿制药获批数量不断增加,推动全球生物仿制药市场增长
美国食品药品管理局 (FDA) 批准生物仿制药,并提供将安全有效的生物仿制药推向市场所需的科学和监管建议。生物仿制药的批准可以增加药物选择的数量,同时降低成本,从而改善患者护理。
下表列出了一些最近批准的生物仿制药产品。
生物仿制药名称
|
批准日期
|
参考产品
|
Alymsys(贝伐单抗)
| 2022 年 4 月 | 阿瓦斯汀(贝伐单抗) |
Cimerli(雷珠单抗-eqrn)
| 2022 年 8 月 | Lucentis(雷珠单抗) |
Fylnetra(聚乙二醇非格司亭-pbbk)
| 2022 年 5 月 | Neulasta(培非格司亭) |
Stimufend(聚乙二醇非格司亭-fpgk)
| 2022 年 9 月 | Neulasta(培非格司亭) |
Vegzelma(贝伐单抗-adcd)
| 2022 年 9 月 | 阿瓦斯汀(贝伐单抗) |
Idacio(阿达木单抗-aacf)
| 2022 年 12 月 | Humira(阿达木单抗) |
Byooviz(雷珠单抗)
| 2021 年 9 月 | Lucentis(雷珠单抗) |
Rezvoglar(甘精胰岛素-aglr)
| 2021 年 12 月 | 来得时(甘精胰岛素) |
Semglee(甘精胰岛素-yfgn)
| 2021 年 7 月 | 来得时(甘精胰岛素) |
Yusimry(阿达木单抗-aqvh)
| 2021 年 12 月 | Humira(阿达木单抗) |
Hulio(阿达木单抗-fkjp)
| 2020 年 7 月 | Humira(阿达木单抗) |
Riabni(利妥昔单抗-arrx)
| 2020 年 12 月 | Rituxan(利妥昔单抗) |
Nyvepria(培非格司亭-apgf)
| 2020 年 6 月 | Neulasta(培非格司亭) |
因此,生物仿制药的批准不断增加正在推动生物仿制药市场的增长。
节段分析
根据疾病适应症,生物仿制药市场细分为癌症、糖尿病、自身免疫性疾病和其他疾病适应症。癌症部分在 2021 年占据最大市场份额,预计自身免疫性疾病在预测期内(2022-2028 年)的复合年增长率最高,为 36.1%。根据药物类别,生物仿制药市场细分为粒细胞集落刺激因子、人类生长激素、胰岛素、TNF 阻滞剂和单克隆抗体以及其他(骨质疏松症等)。粒细胞集落刺激因子药物类别部分在 2021 年占据最大市场份额。此外,预计其他药物类别部分在预测期内的复合年增长率最高。根据应用,全球生物仿制药市场分为静脉注射、皮下注射和其他应用。静脉注射部分在 2021 年占据最大市场份额,预计在预测期内的复合年增长率最高。按最终用户划分,生物仿制药市场分为医院、专科诊所、家庭护理和其他最终用户。医院部门在 2021 年占据了最大的市场份额,预计家庭护理部门在预测期内(2022-2028 年)的复合年增长率最高,为 36.6%。
区域分析
2021 年北美生物仿制药市场价值为 54.7984 亿美元,预计到 2028 年将达到 477.468 亿美元;预计预测期内的复合年增长率为 37.3%。北美生物仿制药市场分为美国、加拿大和墨西哥。2019 年,美国占据北美生物仿制药市场的最大份额。糖尿病和不孕症的发病率不断上升,生物仿制药市场的产品开发也不断增加。根据美国国立卫生研究院自身免疫性疾病协调委员会的数据,2019 年,超过 2400 万美国人患有自身免疫性疾病。八百万人有自身抗体,即血液分子,表明一个人患自身免疫性疾病的风险。由于未知原因,自身免疫性疾病影响着越来越多的人。据美国国家环境健康科学研究所(NIEHS)临床研究部门称,2020年,抗核抗体(ANA)的患病率显著上升,ANA是美国最常见的自身免疫生物标志物。该研究首次评估了美国人口代表性样本中ANA随时间的变化,样本包括男性、非西班牙裔白人、50岁以上的成年人和青少年。在美国,生物仿制药用于治疗癌症、肾病、糖尿病和其他自身免疫性疾病,如类风湿性关节炎和克罗恩病。据康德乐称,美国共有33种生物仿制药获得FDA批准,其中21种已经上市。在上市的21种生物仿制药中,17种用于癌症相关治疗,3种用于治疗自身免疫性疾病,1种用于治疗糖尿病。
生物制剂是美国最昂贵的药物,每位患者每年的费用总计数万美元。生物仿制药的价格预计将比其参考产品低 15% 至 30%。仅在 2020 年,生物仿制药就节省了 79 亿美元,随着更多生物仿制药进入市场,预计未来几年节省的费用将大幅增加。据 Cardinal Health 称,预计到 2025 年,生物仿制药将使美国药品支出减少 1330 亿美元。因此,在美国,生物仿制药具有巨大的潜力,可以降低生物药物的成本,使患者更容易获得治疗,并创造新的创新和科学突破,从而推动该地区生物仿制药市场的增长
关键球员分析
生物仿制药市场分析包括安进公司、赛尔群公司、赛诺菲公司、Biocon 有限公司、辉瑞公司、三星生物epis 有限公司、Coherus BioSciences 公司、礼来公司、山德士公司、梯瓦制药工业有限公司和雷迪博士实验室有限公司等参与者。在生物仿制药市场的参与者中,辉瑞公司和诺华公司凭借提供多样化的产品组合位居前两位。
生物仿制药市场区域洞察
Insight Partners 的分析师已详尽解释了预测期内影响生物仿制药市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的生物仿制药市场细分和地理位置。
- 获取生物仿制药市场的区域特定数据
生物仿制药市场报告范围
| 报告属性 | 细节 |
|---|---|
| 2021 年市场规模 | 184.4亿美元 |
| 2028 年市场规模 | 1360.7亿美元 |
| 全球复合年增长率(2021 - 2028) | 34.8% |
| 史料 | 2019-2020 |
| 预测期 | 2022-2028 |
| 涵盖的领域 | 按疾病指征
|
| 覆盖地区和国家 | 北美
|
| 市场领导者和主要公司简介 |
|
生物仿制药市场参与者密度:了解其对业务动态的影响
生物仿制药市场正在快速增长,这得益于终端用户需求的不断增长,而这些需求又源于消费者偏好的不断变化、技术进步以及对产品优势的认识不断提高等因素。随着需求的增加,企业正在扩大其产品范围,进行创新以满足消费者的需求,并利用新兴趋势,从而进一步推动市场增长。
市场参与者密度是指在特定市场或行业内运营的企业或公司的分布情况。它表明在给定市场空间中,相对于其规模或总市场价值,有多少竞争对手(市场参与者)存在。
在生物仿制药市场运营的主要公司有:
- 比奥康有限公司
- 赛诺菲
- Celltrion 公司
- 安进公司
- 辉瑞公司
免责声明:上面列出的公司没有按照任何特定顺序排列。
- 了解生物仿制药市场顶级关键参与者概况
最新动态
生物仿制药市场中的公司普遍采用并购、产品发布等无机和有机策略。以下列出了一些近期的关键市场发展:
- 2022 年 1 月,Biocon Ltd. 的子公司 Biocon Biologics 完成了对 Viatris 全球生物仿制药业务的收购。此次收购为 Biocon Biologics 提供了在发达市场和几个新兴市场的直接商业能力和支持基础设施,使其更接近患者、客户和付款人。通过此次收购,Biocon Biologics成为拥有八种商业化产品的全球领先生物仿制药公司。
- 2022 年 10 月,Biocon Biologics 将两项生物仿制药资产授权给吉藤制药,在日本进行商业化。根据该交易的条款,吉藤制药获得了由 Biocon Biologics 开发和生产的 bUstekinumab 和 bDenosumab 在日本的独家商业化权利,潜在市场机会为 7 亿美元。
- 2022 年 12 月,Celltrion USA 宣布向 FDA 提交 CT-P13 新型皮下制剂的生物制品许可申请 (BLA)。皮下制剂有可能通过提供高度一致的药物暴露和便捷的给药方法,增强使用英夫利昔单抗药物的治疗选择。
- 2022 年 9 月,Celltrion USA 的肿瘤生物仿制药 Vegzelma 获得美国 FDA 批准,用于治疗六种类型的癌症,例如转移性结直肠癌;复发性或转移性非鳞状非小细胞肺癌 (nsNSCLC);复发性胶质母细胞瘤;转移性肾细胞癌;持续性、复发性或转移性宫颈癌;以及上皮性卵巢癌、输卵管癌或原发性腹膜癌。Vegzelma 是 Celltrion 第三个获得美国 FDA 批准的肿瘤生物仿制药。
- 2022 年 5 月,Biocon Biologics 和 Viatris 推出 Abevmy。Biocon Ltd. 的子公司 Biocon Biologics Ltd. 和 Viatris Inc. 宣布 Abevmy (bBevacizumab) 已在加拿大上市。Abevmy 由 Biocon Biologics 和 Viatris 共同开发,是罗氏 Avastin (Bevacizumab) 的生物仿制药,已获得加拿大卫生部批准用于治疗四种肿瘤适应症。
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST 和 SWOT 分析
- 市场规模价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
- Railway Braking System Market
- Sports Technology Market
- Aircraft Wire and Cable Market
- Rare Neurological Disease Treatment Market
- Lymphedema Treatment Market
- Neurovascular Devices Market
- Sexual Wellness Market
- Virtual Event Software Market
- Non-Emergency Medical Transportation Market
- Aerospace Forging Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends
Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America
Country Scope
This text is related
to country scope.
常见问题
The Asia Pacific is expected to be the fastest-growing region in the Biosimilars market over the forecast period due to the increasing prevalence of chronic diseases and the cost-effectiveness of biosimilars drugs.
The Biosimilars market is estimated to be valued at US$ 22,676.15 million in 2022.
The Biosimilars market is expected to be valued at US$ 1,36,069.53 million in 2028.
Biosimilars are safe and effective treatment options for many illnesses such as chronic skin and bowel diseases (like psoriasis, irritable bowel syndrome, Crohn’s disease and colitis), arthritis, kidney conditions, and cancer. A biosimilar product is a biologic product that is approved based on demonstrating that it is highly similar to an FDAâ€approved biologic product, known as a reference product, and has no clinically meaningful differences in terms of safety and effectiveness from the reference product.
The CAGR value of the biosimilars market during the forecasted period of 2022-2028 is 34.8%.
The Biosimilars market majorly consists of the players, such as Biocon Ltd, Sanofi-Aventis, Celltrion Inc., Amgen Inc., Pfizer Inc., Samsung Bioepis, Sanofi SA, Coherus BioSciences Inc, Dr. Reddy’s Laboratories Ltd, Eli Lilly and Co, Sandoz AG, and Teva Pharmaceutical Industries Ltd.
The factors that are driving the growth of the biosimilars market are the increasing aging population, changing social behavior, and the rising adoption of a sedentary lifestyle by people with accelerating urbanization boost the prevalence of obesity and various chronic diseases, such as diabetes. Also,twin studies have long established that genes can cause chronic conditions such as cardiovascular disease (CVDs), diabetes, obesity, Alzheimer's disease (AD), and depression. These are some of the major factors contributing to the growth of the biosimilars industry.
The insulin segment held the largest share of the market in 2022. Also, the same segment is estimated to register the highest CAGR in the market during the forecast period.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Biosimilar Market
- Biocon Ltd
- Sanofi-Aventis
- Celltrion Inc.
- Amgen Inc.
- Pfizer Inc.
- Samsung Bioepis
- Sanofi SA
- Coherus BioSciences Inc
- Dr. Reddy’s Laboratories Ltd
- Eli Lilly and Co
- Sandoz AG
- Teva Pharmaceutical Industries Ltd.
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published and advised several client across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organization are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:
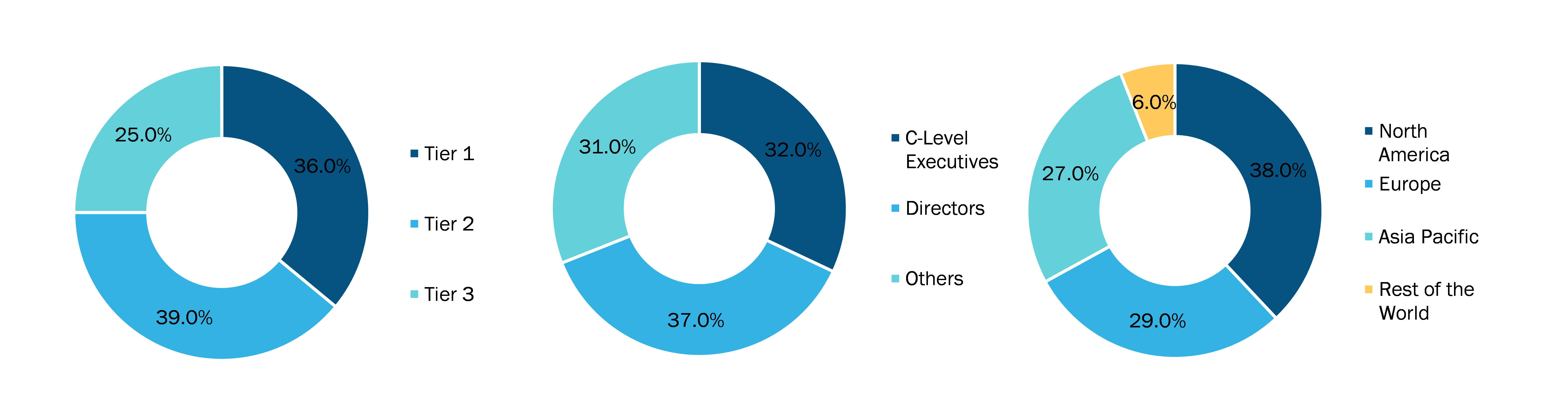
Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
 获取此报告的免费样本
获取此报告的免费样本