页面已更新 :
Jan 2025
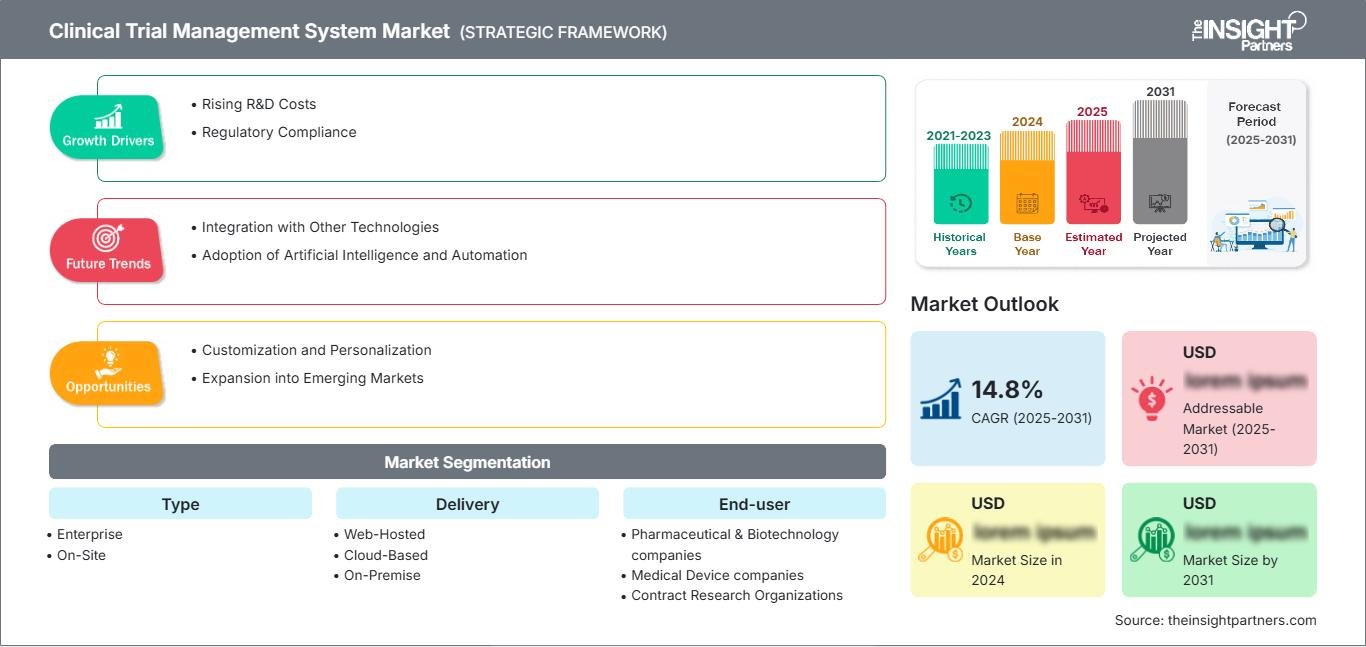
预计临床试验管理系统市场在 2025 年至 2031 年期间的复合年增长率为 14.8%,市场规模将从 2024 年的 XX 百万美元扩大到 2031 年的 XX 百万美元。
该报告按类型(企业和现场);交付(Web 托管、基于云和本地)最终用户(制药和生物技术公司、医疗器械公司、合同研究组织 [CRO] 等)细分。全球分析进一步细分为区域和主要国家。该报告以美元提供了上述分析和细分的价值
报告目的
Insight Partners 撰写的《临床试验管理系统市场》报告旨在描述当前形势和未来增长、主要驱动因素、挑战和机遇。这将为各种商业利益相关者提供见解,例如:
- 技术提供商/制造商:了解不断变化的市场动态并了解潜在的增长机会,使他们能够做出明智的战略决策。
- 投资者:对市场增长率、市场财务预测和整个价值链中存在的机会进行全面的趋势分析。
- 监管机构:规范市场政策和警察活动,旨在最大限度地减少滥用,维护投资者的信任和信心,维护市场的完整性和稳定性。
临床试验管理系统市场细分类型
- 企业
- 现场
交付
- 网络托管
- 基于云
- 本地
最终用户
- 制药和生物技术公司
- 医疗器械公司
- 合同研究组织
地理位置
- 北美
- 欧洲
- 亚太地区
- 中东和非洲
- 南美洲和中美洲
自定义此报告以满足您的要求
您将免费获得任何报告的定制,包括本报告的部分内容,或国家级分析、Excel 数据包,以及为初创企业和大学提供超值优惠和折扣
临床试验管理系统市场: 战略洞察
-
获取本报告的主要市场趋势。这个免费样本将包括数据分析,从市场趋势到估计和预测。
临床试验管理系统市场增长动力
- 研发成本上升:临床试验研发活动的投资不断增加。这一因素促使人们采用能够有效管理临床试验流程的数字解决方案。此外,不断上升的研发成本也鼓励组织采用先进的临床试验管理系统。
- 法规遵从性:日益严格的监管审查和遵守良好临床实践 (GCP) 指南的需求使得必须采用强大的系统来监测和记录试验。
临床试验管理系统市场未来趋势
- 与其他技术的集成:临床试验管理系统将与电子数据采集 (EDC)、电子健康记录 (EHR) 和其他临床系统等技术更加紧密地集成,以创建无缝的工作流程。用于改善结果的集成技术将在未来几年推动市场增长。
- 人工智能和自动化的应用:人工智能和机器学习算法的采用将改善数据管理、患者招募和分析。这一因素使临床试验管理系统更加高效、有效。
临床试验管理系统的市场机遇
- 定制化和个性化:提供针对特定行业需求(例如肿瘤学或罕见疾病)的可定制解决方案,可以为提供商带来竞争优势。为了获得竞争优势,市场参与者可以采用临床试验管理系统。
- 扩展到新兴市场:随着发展中国家的医疗保健系统广泛采用数字解决方案,市场存在增长机会,因为它可以为最终用户提供增强的临床试验服务。
临床试验管理系统市场区域洞察
The Insight Partners 的分析师已详尽阐述了预测期内临床试验管理系统市场的区域趋势和影响因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的临床试验管理系统市场细分和地域分布。
临床试验管理系统市场报告范围
| 报告属性 | 细节 |
|---|---|
| 市场规模 2024 | US$ XX million |
| 市场规模 2031 | US$ XX Million |
| 全球复合年增长率 (2025 - 2031) | 14.8% |
| 历史数据 | 2021-2023 |
| 预测期 | 2025-2031 |
| 涵盖的领域 |
By 类型
|
| 覆盖地区和国家 |
北美
|
| 市场领导者和主要公司简介 |
|
临床试验管理系统市场参与者密度:了解其对业务动态的影响
临床试验管理系统市场正在快速增长,这得益于终端用户需求的不断增长,而这些需求的驱动因素包括消费者偏好的不断变化、技术进步以及对产品优势的认知度不断提高。随着需求的增长,企业正在扩展其产品线,不断创新以满足消费者需求,并利用新兴趋势,从而进一步推动市场增长。
- 获取 临床试验管理系统市场 主要参与者概述
主要卖点
- 全面覆盖:该报告全面涵盖了临床试验管理系统市场的产品、服务、类型和最终用户的分析,提供了整体格局。
- 专家分析:该报告基于对行业专家和分析师的深入了解而编写。
- 最新信息:该报告涵盖了最新信息和数据趋势,确保了业务相关性。
- 定制选项:此报告可以根据特定客户要求进行定制,并恰如其分地适应业务策略。
因此,临床试验管理系统市场研究报告可以帮助引领解读和理解行业情景和增长前景的线索。尽管可能存在一些合理的担忧,但本报告的总体优势往往大于劣势。
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
相关报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势
我们的客户































87-673-9708

ISO 9001:2015





 获取免费样品 - 临床试验管理系统市场
获取免费样品 - 临床试验管理系统市场