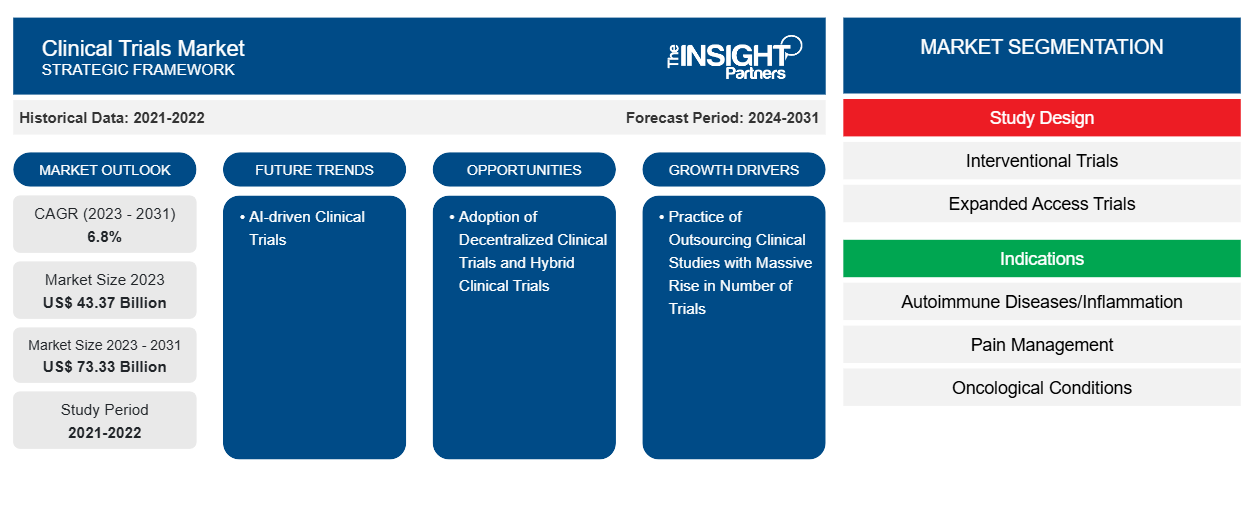
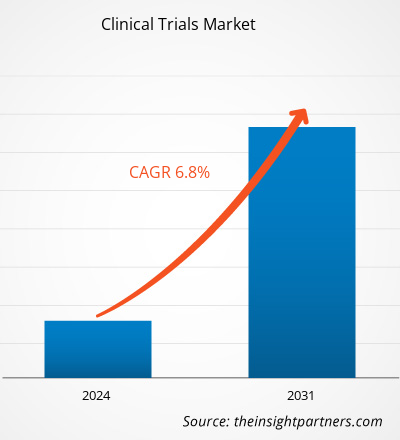
临床试验市场规模预计将从 2023 年的 433.7 亿美元增至 2031 年的 733.3 亿美元。预计 2023-2031 年期间市场复合年增长率为 6.8%。人工智能驱动的临床试验很可能成为未来几年市场的发展趋势。
临床试验市场分析
临床试验市场主要由制药、生物技术和医疗器械公司为产品创新而不断努力所推动。推动市场进步的其他因素包括临床试验的全球化、相关技术的快速发展以及对进行临床试验的 CRO 的需求增加。此外,“2022 年全球临床试验连接”等会议为该市场中的公司提供了一个了解临床试验和临床研究进展的平台。
临床试验市场概览
公司不断增加的战略举措(例如产品发布、并购、合作伙伴关系和地域扩张)使临床试验市场受益。2023 年 9 月,ICON plc 发布了其下一代临床试验代币化解决方案。结合其专有的代币化引擎、对真实世界数据的访问以及先进的临床分析专业知识,ICON 现在提供了一个有凝聚力和无缝的运营模式,在整个产品开发生命周期中提供有关药物安全性和有效性的宝贵、长期见解。2023 年 2 月,Labcorp 宣布计划在计划分拆其临床开发业务后成立一家新公司 Fortrea。从 Labcorp 分拆完成后,Fortrea 将作为一家独立的、公开交易的全球 CRO 运营,提供全面的药物和医疗器械开发服务。
定制此报告以满足您的需求
您可以免费定制任何报告,包括本报告的部分内容、国家级分析、Excel 数据包,以及为初创企业和大学提供优惠和折扣
- 获取此报告的关键市场趋势。这个免费样品将包括数据分析,从市场趋势到估计和预测。
临床试验市场驱动因素和机遇
临床试验外包实践推动市场发展,试验数量大幅增加
临床试验是为了确定一种新型医疗产品(即药物、饮食或医疗器械)是否安全有效。试验主要是整个药物开发过程的一部分。根据美国国家医学图书馆 (NLM) 的数据,2020 年在 NLM (ClinicalTrials.gov) 注册了约 52,000 项新研究,到 2023 年这一数字将增加到约 58,000 项。2023 年 1 月,NLM 报告称美国有 38,837 项活跃临床试验,全球有 105,172 项活跃临床试验。根据欧洲药品管理局的数据,在欧盟 (EU),每年批准约 4,000 项临床试验,其中约 60% 的研究与制药行业有关。临床试验数量的激增可以归因于全球慢性病患病率的上升,这带来了对更有效治疗方法的巨大需求。
临床试验的日益复杂增加了正确执行和监督研究型组织开展的运营的重要性。为了避免因执行不当而导致的错误,许多研究型组织将临床试验外包给临床研究组织 (CRO)。CRO 通过其高质量设施和深厚的专业知识提供的服务协助成功实施临床试验。他们通过高效且具有成本效益的运营逐渐成为临床试验行业的中坚力量,使试验发起人受益。根据赛默飞世尔科技发布的一篇博客,2022 年,CRO 执行了约 4 项临床试验中的 3 项,以保证药物开发商的临床计划,提供丰富的专业知识,提高时间和成本效率,并提供定制的高质量数据。因此,临床试验数量的增加以及将试验外包给 CRO 以提高成本效益和减少错误的做法是推动临床试验市场增长的主要因素。
采用分散临床试验和混合临床试验为市场提供机会
分散式临床试验 (DCT) 的受试者无需频繁访问医院试验地点。在 DCT 中,数字技术用于实现远程数据收集和监控,以及研究人员和参与者之间的简化沟通。混合临床试验方法结合了家庭活动和现场活动,带来最佳患者体验并满足复杂的协议制度,在各个治疗领域和试验阶段旅程中获得了关注。患者隐私、数据安全、监管障碍和复杂的协议制度等挑战阻碍了过去 DCT 的采用。然而,COVID-19 大流行迫使临床试验的赞助商采用分散和混合临床技术来开发药物,因为在健康危机期间无法进行面对面的研究。由于通勤受到限制,收集数据和保持试验进行的唯一方法是远程工作并充分利用技术来加速流程。根据麦肯锡提供的数据,约 70% 的临床试验潜在参与者居住在试验地点之外。因此,分散化扩大了试验的可及性,可以覆盖更多受试者,包括可能更多样化的患者群体。混合临床试验允许申办方将 DCT 元素战略性地纳入研究设计中。这些试验模型提供了前所未有的灵活性;因此,越来越多的公司对混合试验表现出兴趣,这正在重新定义行业格局。据 ObvioHealth 称,FDA 计划在 2023 年公布支持使用 DCT 方法的协议,以提高未来临床研究的可信度。因此,与传统临床试验方法相比,人们越来越关注使用分散和混合临床试验,预计将在预测期内为临床试验市场提供丰厚的机会。
临床试验市场报告细分分析
有助于临床试验市场分析的关键部分是研究设计、适应症和阶段。
- 根据研究设计,临床试验市场分为介入试验和扩大准入试验。2023 年,介入试验领域占据了更大的市场份额。
- 根据适应症,市场细分为心血管疾病、肿瘤疾病、神经系统疾病、自身免疫性疾病/炎症、疼痛管理、糖尿病、肥胖症、代谢紊乱等。肿瘤疾病领域在 2023 年占据了最大的市场份额。
- 根据阶段,临床试验市场分为 I 期、II 期和 III 期。II 期部分在 2023 年占据了最大的市场份额。
临床试验市场份额按地区分析
临床试验市场报告的地理范围主要分为五个地区:北美、亚太地区、欧洲、南美和中美以及中东和非洲。北美在 2023 年占据了市场主导地位。美国是临床试验规模最大、增长最快的市场。美国正在成为领先的临床研究目的地,各公司都在提供创新的临床试验服务。美国已成为领先的临床研究目的地。近一半的临床试验是在美国进行的。此外,大多数制药研究公司更愿意在美国进行临床试验,因为该国拥有完善的医疗基础设施、快速的审批时间表、有利的监管框架以及全球接受的临床试验生成的数据。世界卫生组织 (WHO) 的一份报告指出,美国的临床试验数量最多,2021 年进行了 157,618 次试验。
临床试验市场区域洞察
Insight Partners 的分析师已详尽解释了预测期内影响临床试验市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的临床试验市场细分和地理位置。
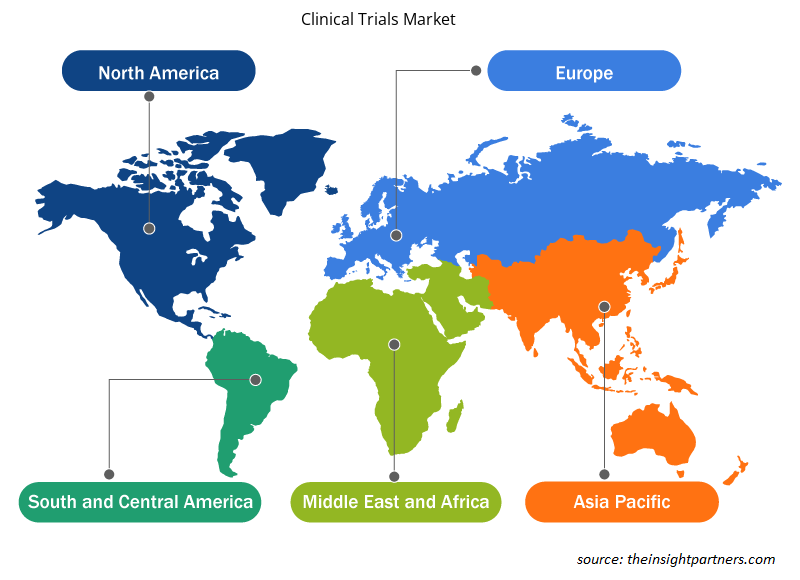
- 获取临床试验市场的区域特定数据
临床试验市场报告范围
| 报告属性 | 细节 |
|---|---|
| 2023 年的市场规模 | 433.7亿美元 |
| 2031 年市场规模 | 733.3亿美元 |
| 全球复合年增长率(2023 - 2031) | 6.8% |
| 史料 | 2021-2022 |
| 预测期 | 2024-2031 |
| 涵盖的领域 | 通过研究设计
|
| 覆盖地区和国家 | 北美
|
| 市场领导者和主要公司简介 |
|
市场参与者密度:了解其对商业动态的影响
临床试验市场正在快速增长,这得益于最终用户需求的不断增长,而这些需求又源于消费者偏好的不断变化、技术进步以及对产品优势的认识不断提高等因素。随着需求的增加,企业正在扩大其产品范围,进行创新以满足消费者的需求,并利用新兴趋势,从而进一步推动市场增长。
市场参与者密度是指在特定市场或行业内运营的企业或公司的分布情况。它表明在给定市场空间中,相对于其规模或总市场价值,有多少竞争对手(市场参与者)存在。
在临床试验市场运营的主要公司有:
- IQVIA 控股公司
- 精鼎国际公司
- IXICO 股份有限公司
- 查尔斯河实验室公司
- ICON 股份有限公司
- 药明康德
免责声明:上面列出的公司没有按照任何特定顺序排列。
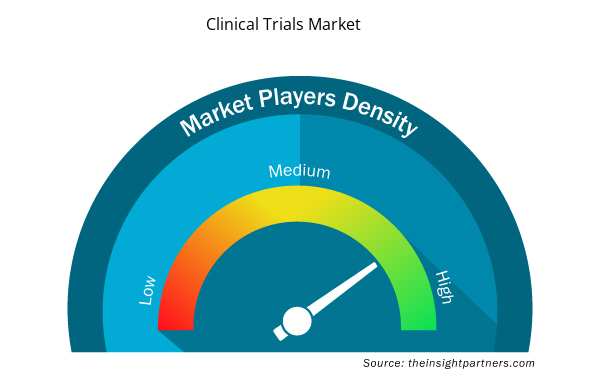
- 了解临床试验市场顶级关键参与者概况
临床试验市场新闻和最新发展
临床试验市场通过收集一手和二手研究后的定性和定量数据进行评估,其中包括重要的公司出版物、协会数据和数据库。以下列出了市场中的一些发展情况:
- Oracle 宣布了 Oracle Clinical One 随机化和试验供应管理 (RTSM) 的新功能。借助使用、访问和区域化方面的新增强功能,Clinical One RTSM 用户可以满足动态的、特定于国家/地区的监管要求,并在从开始到结束的试验中获得速度、可靠性和透明度。(来源:Oracle Corp,公司网站,2024 年 5 月)
- Parexel 与领先的人工智能 (AI) 系统制造商 Palantir Technologies Inc. 达成多年战略合作伙伴关系,利用人工智能帮助全球范围内增强、加速、安全和有效地进行临床试验。在此次合作下,Parexel 利用 Palantir 的 Foundry 和人工智能平台 (AIP) 为其临床数据平台提供支持,该平台专注于提高临床试验效率,同时保持最高水平的安全性和监管严谨性。(来源:Parexel International Corp,公司网站,2024 年 4 月)
临床试验市场报告覆盖范围和交付成果
“临床试验市场规模和预测(2021-2031)”报告对以下领域进行了详细的市场分析:
- 临床试验市场规模及全球、区域和国家层面所有主要细分市场的预测
- 临床试验市场趋势以及市场动态,如驱动因素、限制因素和关键机遇
- 详细的 PEST 和 SWOT 分析
- 临床试验市场分析涵盖主要市场趋势、全球和区域框架、主要参与者、法规和最新市场发展
- 行业格局和竞争分析,涵盖市场集中度、热图分析、知名参与者以及临床试验市场的最新发展
- 详细的公司简介
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST 和 SWOT 分析
- 市场规模价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends
Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America
Country Scope
This text is related
to country scope.
常见问题
IQVIA Holdings Inc, Parexel International Corporation, IXICO Plc, Charles River Laboratories Inc, ICON Plc, WuXi AppTec Co Ltd, SGS SA, Syneos Health Inc, SIRO Clinpharm Private Limited, Thermo Fisher Scientific Inc, Laboratory Corp of America Holdings, CliniRx, Caidya, Oracle Corp, and Medpace are among the key players in the market.
AI-driven clinical trials are expected to emerge as a prime trend in the market in the coming years.
The clinical trials market value is expected to reach US$ 73.33 billion by 2031.
The market is expected to register a CAGR of 6.8% during 2023–2031.
North America dominated the market in 2023.
The practice of outsourcing clinical studies with a massive rise in the number of trials and the flourishing pharmaceutical industry with a surge in R&D activities are among the most significant factors fueling the market growth.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Clinical Trials Market
- IQVIA Holdings Inc
- Parexel International Corp
- IXICO Plc
- Charles River Laboratories International Inc
- ICON Plc
- WuXi AppTec Co Ltd,
- SGS SA
- Syneos Health Inc
- SIRO Clinpharm Pvt Ltd
- Thermo Fisher Scientific Inc
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published and advised several client across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organization are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:
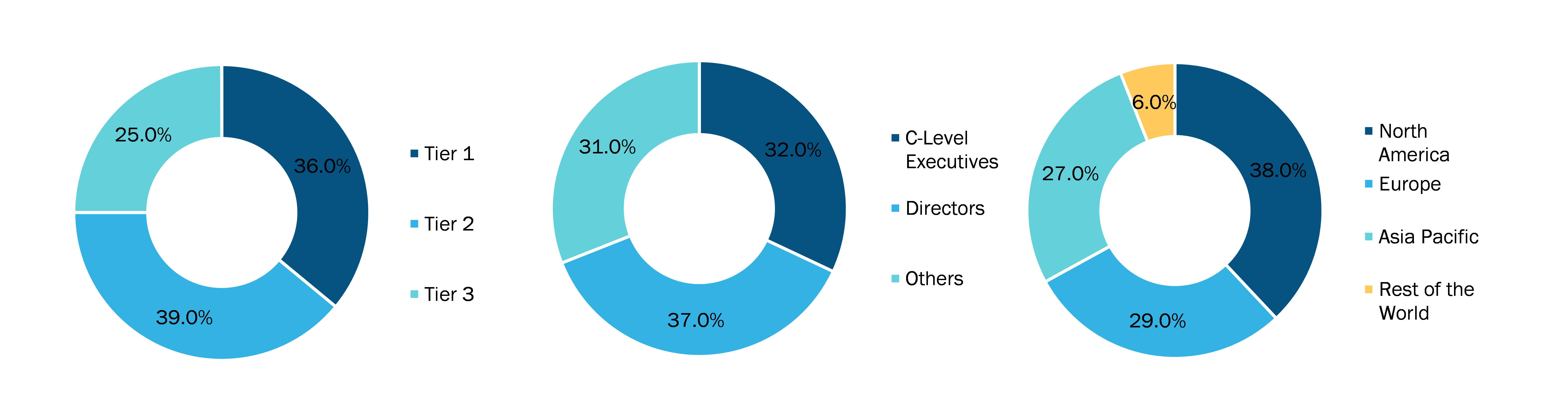
Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
 获取此报告的免费样本
获取此报告的免费样本