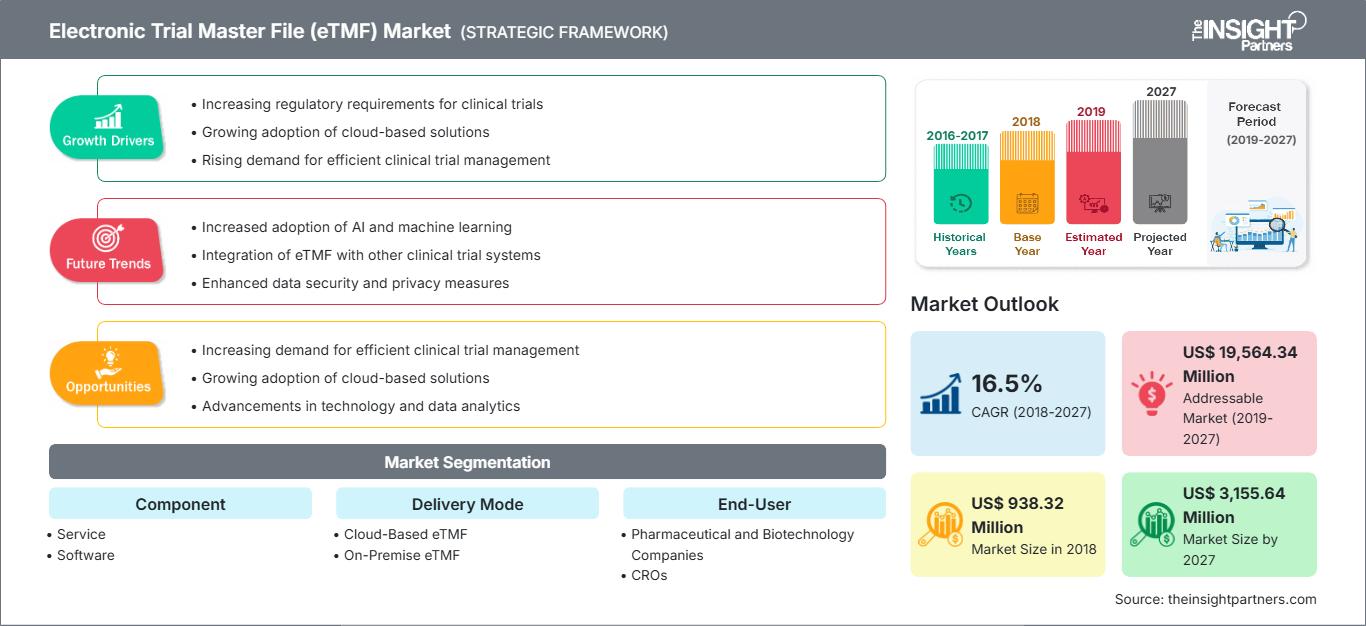
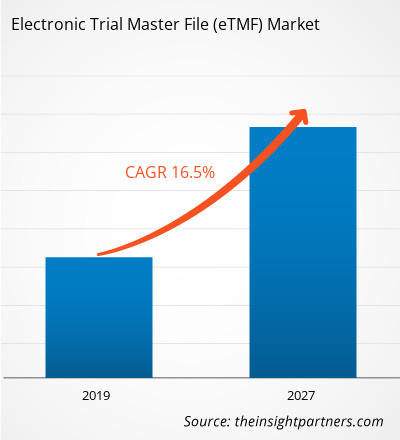
[研究报告]2018 年,医疗保健市场中的电子试验主文件 (eTMF) 价值为 9.3832 亿美元,预计到 2027 年将达到 31.5564 亿美元;预计 2019 年至 2027 年的复合年增长率为 16.5%。
电子试验主文件 (eTMF) 系统可以定义为软件和硬件组件的集成,共同负责临床试验数据的最佳管理。这些解决方案有助于以易于存储的数字格式简化临床试验过程中生成的数据,不同用户可以检索这些数据,从而易于访问并降低与临床试验中的管理和手动数据维护操作相关的成本。医疗保健市场中电子试验主文件的增长归因于临床试验数量的增加、疾病患病率的上升和技术进步多年来推动了市场的发展。然而,缺乏熟练的专业人员可能会对未来几年的市场增长产生负面影响。另一方面,市场参与者不断增加的战略举措可能会在未来几年提供增长机会。
预计医疗保健市场的电子试验主文件 (ETR) 在疫情后将出现大幅增长。由于封锁、旅行禁令和企业倒闭,COVID-19 已影响到各国的经济和行业。COVID-19 危机使许多国家的公共卫生系统不堪重负,凸显了对卫生系统可持续投资的强烈需求。随着 COVID-19 疫情的进展,预计医疗保健行业的增长将下降。由于对体外诊断产品的需求增加以及全球研发活动的增加,生命科学领域蓬勃发展。然而,由于手术数量减少以及设备采购推迟或延长,医疗技术和成像领域的销售额正在下降。此外,预计医疗保健专业人员的虚拟咨询将成为疫情后主流的医疗服务模式。随着远程医疗彻底改变医疗服务方式,数字医疗将在未来几年继续蓬勃发展。此外,临床试验的中断以及随之而来的药物上市延迟,预计也将为未来完全虚拟的试验铺平道路。mRNA 等新技术预计将出现并改变制药行业,预计未来几年市场也将见证更多的垂直整合和合资企业。
自定义此报告以满足您的要求
您将免费获得任何报告的定制,包括本报告的部分内容,或国家级分析、Excel 数据包,以及为初创企业和大学提供超值优惠和折扣
电子审判主文件(eTMF)市场: 战略洞察
-
获取本报告的主要市场趋势。这个免费样本将包括数据分析,从市场趋势到估计和预测。
研发 (R&D) 是公司业务的重要组成部分。制药行业的运营以研发和制造投资的形式对社会产生重大的社会经济影响。研发是任何药物发现系统成功的“支柱”,而电子试验主文件是基于新药和生物技术的治疗实体研发的重要软件。制药和生物技术公司主要致力于研发 (R&D),以开发用于各种治疗应用且具有最大医疗和商业潜力的新分子。这些公司主要投资于研发,旨在向市场提供高质量和创新的产品。例如,2017 年全球研发支出与 2016 年相比增长了 3.9%,达到 1650 亿美元。平均研发支出占处方药总销售额的比例小幅增长至 20.9%。此外,根据美国药物研究和制造商协会 (PhRMA) 2017 年的年度调查,制药公司报告在研发上的支出为 714 亿美元。
制药公司在研发方面投入了更多资金,以加快临床试验流程。例如,截至 2019 年 6 月 30 日,阿斯利康开辟了先河,将 25.63% 的收入用于研发;截至 2019 年 3 月 31 日,礼来公司保持强劲,其将 22.38% 的收入用于研发;截至 2019 年 6 月 30 日,罗氏控股公司紧随其后,其研发支出为 21.29%。
研发支出通常发生在发现、测试和开发新产品、预付款和里程碑、改进现有成果以及在上市前展示产品功效和法规遵从性的过程中。此外,美国制药公司的研发投入在过去15年中持续增长。
药物开发和发现是一个耗时且昂贵的过程。从早期检测或设计到开发再到监管部门批准的过程可能需要10到15年以上。在药物的整个开发阶段,需要各种测试服务来检查产品的质量和功效。因此,制药和生物技术公司倾向于将数据保存到电子主文件中,以节省成本和时间,预计这将推动市场的增长。
临床试验是药物发现中最重要和最有意义的步骤之一,无论治疗方法、医疗策略或设备对人类和兽医用途是否安全有效。临床研究有助于理解和确定特定治疗领域的最佳治疗方法。临床试验旨在收集新产品和新工具开发安全性和有效性的数据。在监管机构批准药物分子和医疗器械之前,需要进行一系列临床研究。各种传染性和非传染性疾病的日益流行,增加了开发用于治疗的新药或医疗器械的需求。这反过来又有望增加各治疗领域对临床试验活动的需求。
参与临床试验的生物制药和制药公司旨在从文件柜中的纸质文档管理系统转向电子文档管理系统,将文档存储在在线电子档案中。通过实施全面的电子临床试验主文件系统 (eTMF) 系统,组织可以自动化、捕获和管理临床试验主文件系统 (TMF) 文档,记录不必要的风险,并且通常可以节省临床试验成本,而无需手动处理纸张。
临床过程中越来越多地采用电子试验主文件系统 (eTMF),这可能会促进市场的发展。例如,Aurea Software 的 NextDocs 是一个电子试验主文件 (eTMF) 论坛,用于在临床试验记录管理中进行临床合作。它是制药行业的内容管理系统,提供一种正式的方式来组织和存储可能需要遵守政府监管机构规定的临床药物试验文件、照片和其他数字内容。在临床试验中,EMA 完全支持使用 eTMF 系统进行电子存储以替代纸质文件。该机构在一份警告声明中指出,由于纸质内容和诸如缺页、标签不当或文档不完整等不一致问题,TMF 和 eTMF 存在质量问题。由于 eTMF 具有临床试验文档的集中和管理、强大的搜索功能(使用元数据)以及多种添加文档的方法等创新功能,eTMF 对于提高业务效率、节约成本以及缩短生物制药产品生产时间以实施电子文档管理流程变得至关重要。引入可互操作的 eTMF 框架的秘诀是使用通用内容模型;基于词汇的标准;以及基于网络的标准技术。
由于试验主文件 (TRMF) 向电子试验主文件 (ETMF) 的进步,合同研究组织 (CRO) 和制药与生物技术公司正在采用 eTMF 来更好地管理临床数据和临床试验管理流程。上述原因和因素促进了电子试验主文件市场的增长。
基于组件的洞察
就组件而言,医疗保健市场中的电子试验主文件 (ETMF) 分为服务和软件。服务部门在 2019 年占据了最大的市场份额。
基于交付模式的洞察
根据交付模式,医疗保健市场中的电子试验主文件 (ETMF) 分为基于云的 etmf 和本地 etmf。 2019年,基于云端的eTMF细分市场占据了最大的市场份额。
基于最终用户的洞察
就最终用户而言,医疗保健市场中的电子试验主文件(eTMF)细分市场可分为制药和生物技术公司、CRO(商业关系组织)和其他公司。制药和生物技术公司细分市场在2019年占据了最大的市场份额。
医疗保健市场中的电子试验主文件(eTMF)参与者正在采用产品发布和扩展策略,以满足全球不断变化的客户需求,这也使他们能够在全球范围内维护自己的品牌。
电子审判主文件 (eTMF) 市场
The Insight Partners 的分析师已详尽阐述了预测期内影响电子审判主文件 (eTMF) 市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的电子审判主文件 (eTMF) 市场细分和地域分布。
电子审判主文件 (eTMF) 市场报告范围
| 报告属性 | 细节 |
|---|---|
| 市场规模 2018 | US$ 938.32 Million |
| 市场规模 2027 | US$ 3,155.64 Million |
| 全球复合年增长率 (2018 - 2027) | 16.5% |
| 历史数据 | 2016-2017 |
| 预测期 | 2019-2027 |
| 涵盖的领域 |
By 组件
|
| 覆盖地区和国家 |
北美
|
| 市场领导者和主要公司简介 |
|
电子审判主文件 (eTMF) 市场参与者密度:了解其对业务动态的影响
电子审判主文件 (eTMF) 市场正在快速增长,这得益于终端用户需求的不断增长,而这些需求的驱动因素包括消费者偏好的演变、技术进步以及对产品优势的认知度的提升。随着需求的增长,企业正在扩展其产品线,不断创新以满足消费者需求,并利用新兴趋势,从而进一步推动市场增长。
- 获取 电子审判主文件(eTMF)市场 主要参与者概述
医疗保健市场中的电子试验主文件 (ETR) — 按组件
- 服务
- 软件
医疗保健市场中的电子试验主文件 (ETR) — 按交付模式
- 基于云的 eTMF
- 本地 eTMF
医疗保健市场中的电子试验主文件 (ETR) — 按最终用户
- 制药和生物技术公司
- 合同研究组织 (CRO)
- 其他
医疗保健市场中的电子试验主文件 (ETR) —按地理位置
-
北美洲
- 美国
- 加拿大
- 墨西哥
-
欧洲
- 法国
- 德国
- 意大利
- 英国
- 西班牙
- 其他地区欧洲
-
亚太地区(APAC)
- 中国
- 印度
- 韩国
- 日本
- 澳大利亚
- 亚太地区其他地区
-
中东和中东地区非洲(MEA)
- 南非
- 沙特阿拉伯
- 阿联酋
- 中东和非洲其他地区
-
南美洲和中美洲(SCAM)
- 巴西
- 阿根廷
- 其他地区SCAM
公司简介
- Aurea, Inc.
- Transperfect。
- Covance Inc(Lab Corp)
- Oracle
- Ennov
- Mastercontrol, Inc.
- Omnicomm
- Pharmavigilalnce
- Veeva Systems
- Phlexglobal
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势






















 获取免费样品 - 电子审判主文件(eTMF)市场
获取免费样品 - 电子审判主文件(eTMF)市场