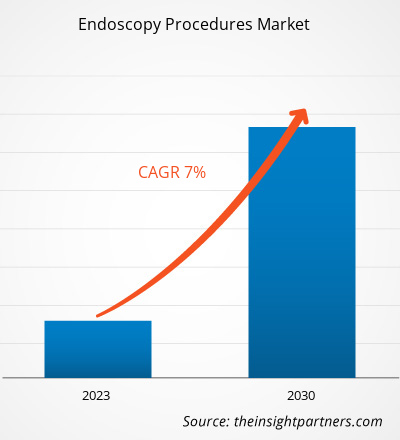
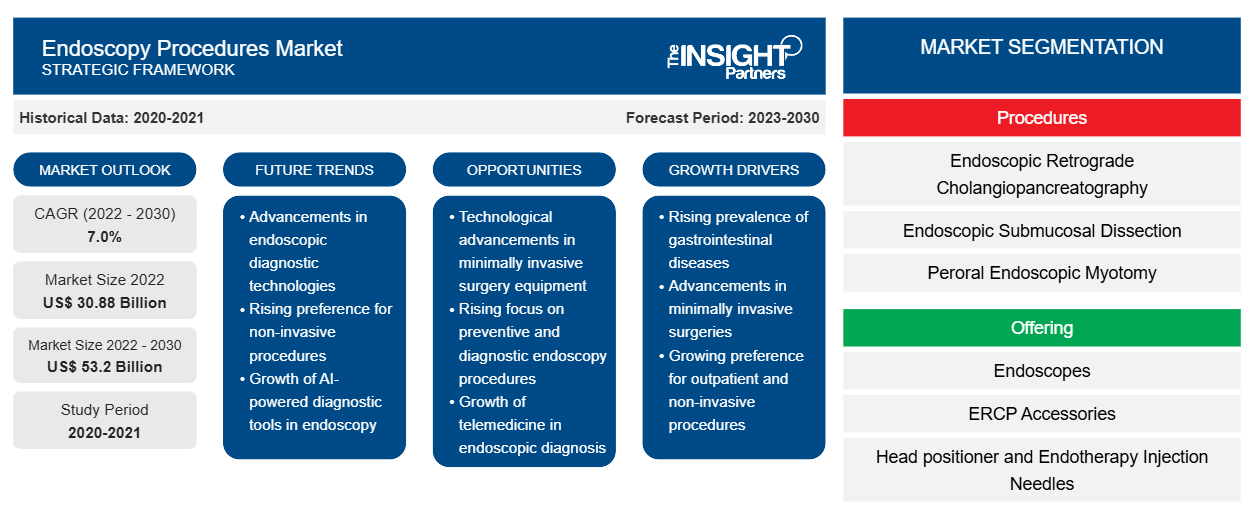
[研究报告] 2022 年内窥镜检查市场规模价值 308.7737 亿美元,预计到 2030 年将达到 531.9665 亿美元。预计 2022-2030 年期间的复合年增长率为 7.0%。
市场洞察和分析师观点:
内窥镜检查是一种微创医疗技术,涉及通过自然开口或小切口将细而柔韧的管子插入体内。这些程序允许医生观察和操作内部器官、组织和结构,而无需进行大手术。内窥镜主要用于诊断和治疗胃肠道、呼吸系统等领域的各种疾病。推动内窥镜检查市场增长的因素是对微创手术的日益偏好和癌症患病率的上升。内窥镜机器的成本可能因内窥镜的类型、设备的复杂性、品牌和其提供的功能而有很大差异。内窥镜检查相关的感染风险可能会在预测期内阻碍市场的增长。
增长动力:
癌症是全球主要公共卫生问题,也是北美的主要死亡原因。北美胃肠道疾病和癌症病例的增加也加速了内窥镜的采用和市场的增长。根据美国癌症协会的数据,结直肠癌是美国第二大死亡原因。男性在一生中患上这种疾病的风险为 4.3%,女性为 4.0%。美国癌症协会预计,2022 年美国将有近 52,580 人死于结直肠癌。
根据 Globocan 2020 的数据,2020 年加拿大胃癌发病率为 3,505,预计到 2040 年将达到 5,230。根据加拿大统计局的数据,肺癌是加拿大最常见的癌症,也是加拿大死亡的主要原因,超过结直肠癌、胰腺癌和乳腺癌的总和。仅在 2021 年,就有约 21,000 名加拿大人死于肺癌。肺癌的高死亡率反映了其低存活率。
肺癌发病率和死亡率随年龄增长而急剧上升。发病率在 75-84 岁加拿大人中达到峰值(每 100,000 人 396 人),而死亡率主要出现在 85 岁及以上的加拿大人中(每 100,000 人 366 人)。总体而言,男性肺癌发病率比女性高 1/10,男性死亡率比女性高近 1/3。然而,对于 55 岁以下的加拿大人来说,女性的发病率高于男性。
美国和加拿大等西方国家报告称,由于成年人口肥胖率上升和膳食纤维摄入量减少,胃肠道疾病发病率较高。根据美国疾病控制和预防中心的数据,2022 年 12 月,美国医生办公室记录了约 3720 万例消化系统疾病病例。
IBD 是指两种疾病——溃疡性结肠炎和克罗恩病。此外,在过去几年中,由于久坐的生活方式、饮食习惯的改变、压力以及不良的营养选择(包括大量摄入超加工食品和反式脂肪),全球 IBD 病例有所增加。根据美国克罗恩病和结肠炎基金会的数据,美国每年诊断出约 70,000 例新的 IBD 病例。此外,由 IBD 引起的慢性炎症和免疫监视能力下降可能导致胃癌的发展。
加拿大癌症负担的不断增加表明对内窥镜设备的需求很高,从而推动了市场的发展。
定制此报告以满足您的需求
您可以免费定制任何报告,包括本报告的部分内容、国家级分析、Excel 数据包,以及为初创企业和大学提供优惠和折扣
-
获取此报告的关键市场趋势。这个免费样品将包括数据分析,从市场趋势到估计和预测。
报告细分和范围:
“全球内窥镜检查程序市场”根据程序、产品、产品类型和最终用户进行细分。根据手术程序,市场分为内镜逆行胰胆管造影术 (ERCP)、内镜黏膜下剥离术 (ESD)、经口内镜肌切开术 (POEM)、内镜超声 (EUS)、介入性肺病学和腹腔镜检查、关节镜和支气管镜检查、结肠镜和阴道镜检查、直肠镜和胸腔镜检查等。在产品方面,市场细分为内窥镜、ERCP 配件、可视化系统、头部定位器和内镜治疗注射针、取样装置和装置夹和电外科刀、内镜超声引导装置、导丝、镊子、圈套器、冲洗/充气管系统、探针、止血夹、息肉陷阱、一次性阀门、套管针套和组织剪刀和切割器、检索装置等。内窥镜检查程序市场按产品类型分为一次性和可重复使用。内窥镜检查程序市场按最终用户分为医院和诊所、门诊手术中心、诊断实验室等。内窥镜检查程序市场按地理位置分为北美(美国、加拿大和墨西哥)、欧洲(德国、法国、意大利、英国、俄罗斯和欧洲其他地区)、亚太地区(澳大利亚、中国、日本、印度、韩国和亚太其他地区)、中东和非洲(南非、沙特阿拉伯、阿联酋和中东和非洲其他地区)以及南美洲和中美洲(巴西、阿根廷和南美洲和中美洲其他地区)。
节段分析:
根据程序,内窥镜检查程序市场细分为内窥镜逆行胰胆管造影术 (ERCP)、内窥镜黏膜下剥离术 (ESD)、经口内窥镜肌切开术 (POEM)、内窥镜超声 (EUS)、介入性肺病学和腹腔镜检查、关节镜和支气管镜检查、结肠镜和阴道镜检查、直肠镜和胸腔镜检查等。2022 年,关节镜和支气管镜检查领域占据了最大的市场份额。内窥镜逆行胰胆管造影术 (ERCP) 领域预计将在预测期内以最快的速度增长。关节镜检查是一种微创内窥镜,用于诊断和手术治疗关节损伤。其本质在于通过 2 个小切口将关节镜插入关节腔,使外科医生能够全面检查关节,获取有关其状况的信息并确定是否存在关节内损伤。关节镜检查允许对膝关节损伤进行关节镜治疗。
这项技术可以去除半月板受损部分,修复韧带、受损软骨并进行许多其他外科手术。支气管镜检查是一种内窥镜检查,使用支气管镜检查上、下呼吸道(气管、支气管)。
根据产品类型,市场细分为内窥镜、ERCP 配件、可视化系统、头部定位器和内镜治疗注射针、取样装置和装置夹以及电外科刀、内窥镜超声引导装置、导丝、镊子、圈套、灌溉/充气管系统、探针、止血夹、息肉陷阱、一次性阀门、套管针套和组织剪刀和切割器、检索设备等。2022 年,内窥镜部分占据了最大的市场份额。ERCP 配件部分预计将在预测期内以最快的速度增长。
根据最终用户,市场分为医院和诊所、门诊手术中心、诊断实验室等。2022 年,医院和诊所部门占据了最大的市场份额。诊断实验室部门预计在预测期内将以最快的速度增长。医院和诊所会进行几种内窥镜检查,可用于诊断和治疗消化系统疾病,包括消化性溃疡、息肉、癌症以及由结石、炎症和肿瘤引起的胆管阻塞。内窥镜诊所专门治疗肠道出血问题、巴雷特食管等癌前异常和家族性腺瘤性息肉病综合征。诊所还专门评估和开发新型内窥镜和内窥镜技术。因此,上述因素正在推动内窥镜检查市场医院和诊所部门的增长。
区域分析:
根据地理位置,内窥镜检查市场分为五个主要区域:北美、欧洲、亚太地区、南美和中美以及中东和非洲。北美市场分析主要集中在三个主要国家——美国、加拿大和墨西哥。预计在预测期内,美国将占据最大的内窥镜检查市场份额。预计不断增长的医疗保健支出将增加各种患者护理设备的采用,从而推动对内窥镜检查产品的需求。根据 2020 年 10 月发布的《全球疾病负担研究》,美国各种疾病负担沉重,例如结直肠癌、肝硬化、慢性肾病、心脏病和癌症。此外,根据美国卫生与公众服务部的数据,2021 年,慢性肾病 (CKD) 影响了七分之一的美国成年人,即估计有 3700 万美国人。美国内窥镜检查市场最近见证了多项技术进步和突破。美国拥有众多全球内窥镜产品制造商,例如波士顿科学公司、宾得医疗、史赛克公司和 Steris 公司。因此,美国内窥镜产品的批准推动了市场的增长。2023 年 2 月,波士顿科学公司获得美国食品药品监督管理局 (FDA) 510(k) 批准 LithoVue Elite 一次性数字柔性输尿管镜系统,这是首个能够在输尿管镜检查过程中实时监测肾内压的输尿管镜系统。因此,随着外科手术的激增,对设施和设备消毒的需求也随之增加。
内窥镜检查市场区域洞察
Insight Partners 的分析师已详尽解释了预测期内影响内窥镜检查程序市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的内窥镜检查程序市场细分和地理位置。
- 获取内窥镜检查程序市场的区域特定数据
内窥镜检查程序市场报告范围
| 报告属性 | 细节 |
|---|---|
| 2022 年市场规模 | 308.8亿美元 |
| 2030 年市场规模 | 532亿美元 |
| 全球复合年增长率(2022 - 2030 年) | 7.0% |
| 史料 | 2020-2021 |
| 预测期 | 2023-2030 |
| 涵盖的领域 |
按程序
|
| 覆盖地区和国家 |
北美
|
| 市场领导者和主要公司简介 |
|
内窥镜检查市场参与者密度:了解其对业务动态的影响
内窥镜检查市场正在快速增长,这得益于终端用户需求的不断增长,而这些需求又源于消费者偏好的不断变化、技术进步以及对产品优势的认识不断提高等因素。随着需求的增加,企业正在扩大其产品范围,进行创新以满足消费者的需求,并利用新兴趋势,从而进一步推动市场增长。
市场参与者密度是指在特定市场或行业内运营的企业或公司的分布情况。它表明在给定市场空间中,相对于其规模或总市场价值,有多少竞争对手(市场参与者)存在。
在内窥镜检查程序市场运营的主要公司有:
- 史赛克公司
- 富士胶片控股公司
- 梅里特医疗系统公司
- 史密斯和侄子公司
- Arthrex公司
免责声明:上面列出的公司没有按照任何特定顺序排列。
- 了解内窥镜检查程序市场顶级关键参与者概况
内窥镜检查程序市场机会:
北美内窥镜领域见证了各种技术进步和产品发布。这些进步不仅有望帮助准确诊断疾病,而且还有助于提高内窥镜检查程序的安全性。以下列出了内窥镜设备市场的一些最新技术进步和产品发布:
- 2021 年 4 月,奥林巴斯宣布其美国支气管镜产品组合中新增了获得 510(k) 认证的 H-SteriScope 一次性支气管镜,这是一系列五款高级内窥镜,用于先进的诊断和治疗程序。H-SteriScope 产品组合的推出是奥林巴斯全资子公司 Veran Medical Technologies, Inc. 与湖南华欣医疗器械有限公司合作的成果。
- 2021 年 4 月,Ambu Inc. 获得了加拿大卫生部对 aScope 4 Cysto 的授权,这是该公司独特的泌尿科柔性膀胱镜平台。Ambu 的 aScope 4 Cysto 是一款经济高效、高品质的柔性内窥镜,具有出色的可操作性和光学性能。与传统的可重复使用内窥镜不同,一次性使用可消除确保每次手术都有患者可用仪器所需的大量资金、维修和清洁成本。每个 aScope 4 膀胱镜从包装中取出后都是无菌的,可随时使用,使医疗机构能够提高工作流程效率,并将员工从复杂、耗时的内窥镜清洁工作中重新分配到更有成效的活动上。
竞争格局和重点公司:
全球内窥镜手术市场中一些知名的内窥镜设备制造商包括 Steris Plc、Conmed Corp、Olympus Corp、Boston Scientific Corp、PENTAX Medical、Erbe Elektromedizin Gmbh、Micro-Tech Nanjing Co Ltd、Merit Medical Systems Inc、Cook Medical LLC、Stryker Corp 和 Johnson & Johnson。这些公司专注于新技术、现有产品的改进和地域扩张,以满足全球不断增长的消费者需求并增加其专业产品组合的产品范围。例如,2022 年 6 月,Stryker 在古尔冈国际科技园开设了新的研发设施,即 Stryker 全球技术中心。这座占地 150,000 平方英尺的设施进一步支持了公司改善医疗保健的使命。
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
相关报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势






















 获取免费样品 - 内窥镜检查市场
获取免费样品 - 内窥镜检查市场