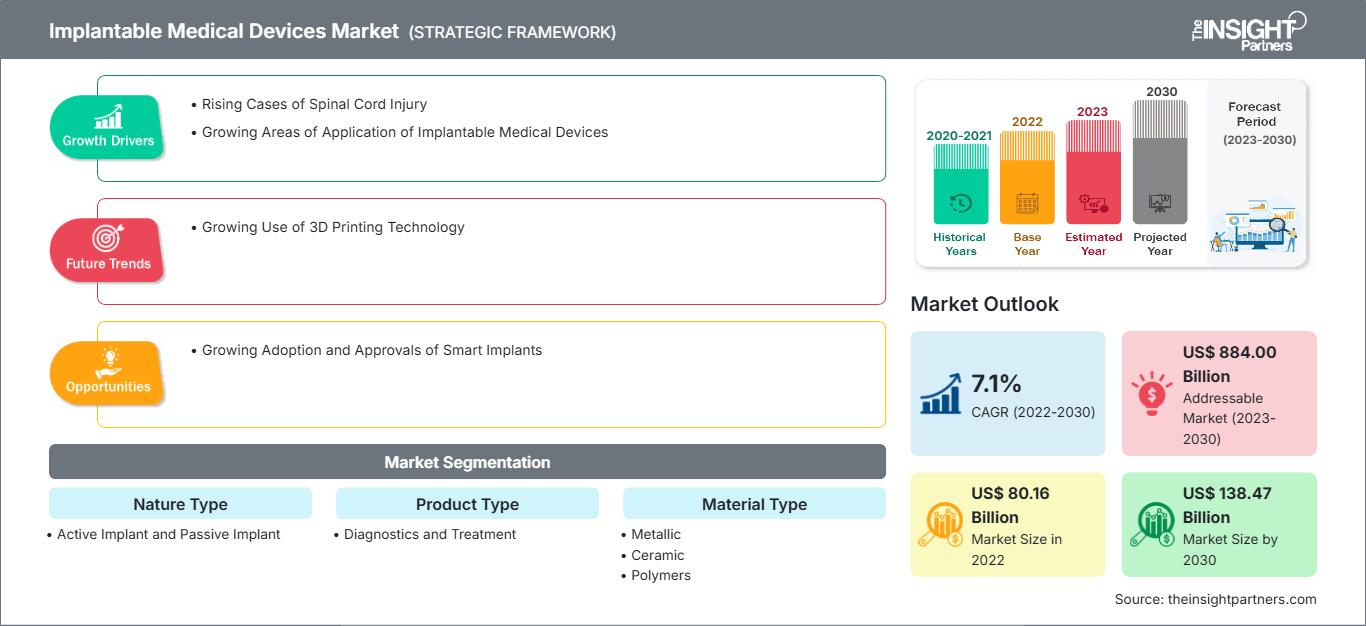
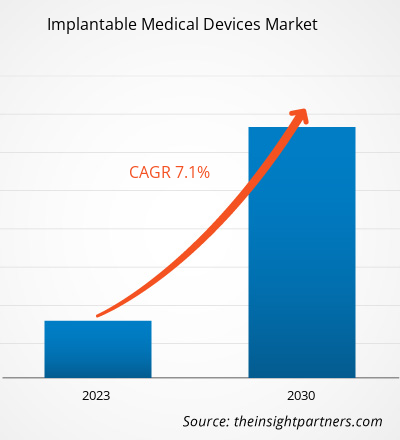
[研究报告] 植入式医疗器械市场预计将从2022年的801.56亿美元增长至2030年的1384.7435亿美元;预计2022年至2030年的复合年增长率为7.1%。
市场洞察与分析师观点:
植入式医疗器械是指完全或部分植入体内的器械。这些医疗器械通常由医生在外科手术过程中植入。与外科医疗器械相比,植入式医疗器械在手术后仍会留在体内。推动植入式医疗器械市场增长的关键因素包括植入式医疗器械应用领域的不断扩大以及脊髓损伤病例的不断增加。
增长动力和制约因素:
据世界卫生组织 (WHO) 统计,2022 年美国记录了 5000 万例癫痫病例、10 亿例偏头痛病例和 40 万例脊髓损伤病例。脊髓刺激是治疗慢性背痛最受欢迎的技术之一,这主要是因为脊髓神经具有控制疼痛感觉的解剖和功能能力。过去十年,脊髓损伤的发病率迅速上升。美国国家脊髓损伤统计中心 (NSCISC) 2021 年情况说明书指出,脊髓损伤的年发病率为每百万 60 例。根据2022年4月发表的《创伤性脊髓损伤流行病学:一项基于人群的大型研究》一文,2021年,创伤性脊髓损伤的年龄-性别标准化发病率为每百万人口26.5例,且无论男女,该发病率均与年龄直接相关。同一来源还指出,在老年人口(65岁及以上)中,男性和女性的发病率分别为每百万人口59.2例和23.3例。脊髓刺激器广泛用于治疗脊柱手术后的疼痛。根据题为《脊髓损伤事实与统计》的报告,2021年,每年有17,700名美国人遭受脊髓损伤,其中78%为平均年龄43岁的男性。因此,老年人和成年人脊髓损伤的高发性刺激了对植入式神经刺激装置的需求,从而推动了市场的增长。然而,植入式医疗器械的使用可以挽救生命,因为这些装置可以减轻疼痛和不适,同时恢复活动能力并改善患者的健康状况。然而,矫形植入手术也存在一些并发症,例如骨折固定、装置故障和关节置换术并发症,包括肩关节、肘关节、髋关节和膝关节脱位。此外,植入物被身体排斥、关节植入物感染、血栓、关节植入物松动以及神经血管损伤都是与矫形外科相关的并发症。植入物。
自定义此报告以满足您的要求
您将免费获得任何报告的定制,包括本报告的部分内容,或国家级分析、Excel 数据包,以及为初创企业和大学提供超值优惠和折扣
植入式医疗器械市场: 战略洞察
-
获取本报告的主要市场趋势。这个免费样本将包括数据分析,从市场趋势到估计和预测。
此外,还有各种用于静脉和鞘内给药以及处理与癌症治疗相关的各种合并症和并发症的装置。其中一些装置包括中心静脉通路装置 (CVAD)、心脏植入式电子装置 (CIED)、Ommaya 储液器、体外心室引流管 (EVD)、乳房植入物加组织扩张器 (TE) 以及经皮肾穿刺管 (PCNT) 等。
与这些装置相关的感染很常见,这会导致医疗成本增加,并增加患者短期和长期肿瘤管理的并发症。这通常会导致进一步的癌症治疗被延迟,直至感染消退。治疗这些感染并更换或移除装置通常是必要的。然而,移除植入物可能很困难,在某些情况下,由于患者潜在的血小板减少症、合并症、缺乏血管通路、免疫抑制以及既往手术干预,移除植入物甚至会令人望而却步。此外,治疗这些感染的报销金额很低。因此,术后并发症阻碍了市场的增长。
报告细分和范围:
全球植入式医疗器械市场根据性质类型、产品类型、材料类型、应用和最终用户进行分类。根据性质类型,植入式医疗器械市场分为主动植入物和被动植入物。根据产品类型,植入式医疗器械分为诊断用和治疗用。根据材料类型,市场细分为金属、陶瓷和聚合物。就应用而言,植入式医疗器械市场细分为心血管植入物、骨科植入物、心血管植入物、乳房植入物、假肢植入物、脑植入物等。就最终用户而言,市场分为医院、专科诊所、ASC 等。根据地域划分,植入式医疗器械市场细分为北美(美国、加拿大和墨西哥)、欧洲(德国、法国、意大利、英国、俄罗斯和欧洲其他地区)、亚太地区(澳大利亚、中国、日本、印度、韩国和亚太其他地区)、中东和非洲(南非、沙特阿拉伯、阿联酋和中东及非洲其他地区)以及南美洲和中美洲(巴西、阿根廷和南美洲和中美洲其他地区)。
分段分析:
植入式医疗器械市场按性质类型细分为主动植入物和被动植入物。 2022年,被动植入物占据了更大的市场份额。然而,预计主动植入物在2022-2030年期间的复合年增长率将更高。被动植入物没有任何电子或磁性元件,也不需要任何外部电源即可工作。被动植入物的一些例子包括导管、电线、动脉瘤夹、支架、外固定装置、髋关节假体和下腔静脉 (IVC) 过滤器。MRI 可能通过加热、旋转、位移和磁化等机制影响被动植入物。
植入式医疗器械市场按产品类型细分为诊断和治疗。治疗领域在2022年占据了更大的市场份额;此外,预计同一领域在2022-2030年期间的复合年增长率将更高。植入式医疗器械被植入体内,用于输送药物并支持特定器官的功能。植入式医疗器械常用于治疗心脏病。此外,各种类型的假体,例如骨科和牙科植入物,都用于替换受损的身体部位。
植入式医疗器械市场按材料类型细分为金属、陶瓷和聚合物。金属植入物在2022年占据了最大的市场份额,而聚合物植入物预计在2022-2030年期间的复合年增长率最高。金属植入物广泛应用于牙科、心脏器械、骨科手术和妇科手术。例如,钴铬钼合金用于骨科植入物和义齿框架。同样,钴及其合金也用于制造除颤器。此外,乙烯-醋酸乙烯酯共聚物 (EVA)、硅胶、聚醚醚酮 (PEEK) 聚合物和超高分子量聚乙烯 (UHMW-PE) 等聚合物由于易于制造、柔韧性和生物相容性而被广泛用于制造医疗植入物。
植入式医疗器械市场按应用细分为牙科植入物、骨科植入物、心血管植入物、乳房植入物、脑植入物等。骨科植入物细分市场在 2022 年占据最大市场份额,预计在 2022-2030 年期间将实现最高复合年增长率。骨科植入物用于替换因畸形或损伤而造成的软骨、骨骼或关节。大多数骨科植入物由钛合金和不锈钢制成,其中一些甚至可能覆盖有塑料。塑料内衬充当人工软骨,而金属结构则为植入物提供必要的强度。通常,植入物被固定到位,支撑骨骼生长,从而提高强度。钛等金属合金是骨科植入物中最常用的假体材料,包括膝关节和髋关节置换术。金属合金也用于骨板和骨螺钉。陶瓷和聚合物是用于合成骨科植入物的其他材料。
植入式医疗器械市场按最终用户细分为医院、专科诊所、美国外科诊所 (ASC) 和其他。2022 年,医院细分市场占据最大市场份额,预计 2022 年至 2030 年期间,医院细分市场的复合年增长率最高。
区域分析:
根据地域分布,全球植入式医疗器械市场分为五个主要区域:北美、欧洲、亚太地区、南美和中美以及中东和非洲。 2022年,北美占据全球植入式医疗器械市场的最大份额。预计亚太地区在2022年至2030年期间的复合年增长率最高。
预计美国将在2022年至2030年期间占据最大的植入式医疗器械市场份额。帕金森病等神经系统疾病发病率的上升、人们对神经系统疾病认识的提高以及对颅内刺激器开发投资的增加,是推动美国整体植入式医疗器械市场发展的主要因素。多巴胺水平低和其他遗传因素是导致帕金森病的主要原因。根据阿尔茨海默病协会发表的一项名为《2022 年阿尔茨海默病事实与数据》的研究,2022 年约有 650 万 65 岁及以上的美国人被诊断患有阿尔茨海默病。预计到 2060 年,这一数字将达到 1380 万。根据脑动脉瘤基金会 2019 年发布的数据,在美国,约有 600 万人患有未破裂的脑动脉瘤。此外,每年的破裂率接近每 10 万人 8-10 人;美国约有 3 万人患有脑动脉瘤破裂。据观察,深部脑刺激 (DBS) 装置可有效控制与帕金森病相关的震颤。
据帕金森病基金会称,美国约有 100 万人患有帕金森病,预计到 2030 年将增至 120 万人。技术进步和新产品的推出推动了植入式医疗器械市场的发展。2020 年 1 月,雅培的 Infinity DBS 系统获得美国食品药品监督管理局 (FDA) 批准,用于治疗帕金森病。该系统可以针对大脑中称为内苍白球 (GPi) 的特定区域进行靶向治疗,该区域与帕金森病的症状相关。因此,神经系统疾病的日益流行和技术的进步推动了美国植入式医疗器械市场的增长。
行业发展和未来机遇:
全球植入式医疗器械市场主要参与者采取的各种举措如下:
- 2023 年 8 月,美敦力公司 (Medtronic plc) 的 Inceptiv 闭环可充电脊髓刺激器 (SCS) 获得了 CE(欧洲合格认证)标志认证。这是第一款提供闭环功能的美敦力 SCS 设备,该功能可感知每个人独特的生物信号并根据需要随时调整刺激,使治疗与日常生活的运动保持协调。
- 2023 年 5 月,BIOTRONIK 宣布其心律管理产品组合中增加了最新的 Amvia Sky 和 Amvia Edge。BIOTRONIK 的最新技术获得了 CE 标志 -全球首批获准用于左束支起搏的起搏器和CRT-P。Amvia Sky 和 Amvia Edge 代表着尖端创新,并融合了最新的心脏病学趋势。
- 2023 年 3 月,登士柏西诺德推出了 DS OmniTaper 种植体系统,这是 EV 种植体系列的最新成员。DS OmniTaper 种植体系统是一项创新解决方案,它将登士柏西诺德 EV 种植体系列的成熟技术与高效多功能的新功能相结合。
- 2023 年 2 月,美敦力公司的 Aurora EV-ICD MRI SureScan(血管外植入式心律转复除颤器)和 Epsila EV MRI SureScan 除颤导线获得了 CE(欧洲合格认证)标志,可用于治疗可能导致心脏骤停的危险快速心律。 Aurora EV-ICD 系统兼具传统 ICD 的救命优势,同时由于其导线(细线)置于心脏和静脉之外,避免了某些风险。
- 2023 年 1 月,雅培宣布美国食品药品监督管理局 (FDA) 已批准其 Proclaim XR 脊髓刺激 (SCS) 系统用于治疗糖尿病疼痛性周围神经病变 (DPN),这是一种使人衰弱的糖尿病并发症。Proclaim XR SCS 系统可以为需要传统治疗方法(例如口服药物)替代方案的 DPN 患者提供缓解。接受 Proclaim XR SCS 系统治疗的患者还可以使用雅培的 NeuroSphere Virtual Clinic,这是一款互联护理应用程序,可帮助患者与医生沟通并远程接受治疗调整。
植入式医疗器械市场区域洞察
The Insight Partners 的分析师已详尽阐述了预测期内影响植入式医疗器械市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的植入式医疗器械市场细分和地域分布。
植入式医疗器械市场报告范围
| 报告属性 | 细节 |
|---|---|
| 市场规模 2022 | US$ 80.16 Billion |
| 市场规模 2030 | US$ 138.47 Billion |
| 全球复合年增长率 (2022 - 2030) | 7.1% |
| 历史数据 | 2020-2021 |
| 预测期 | 2023-2030 |
| 涵盖的领域 |
By 自然类型
|
| 覆盖地区和国家 |
北美
|
| 市场领导者和主要公司简介 |
|
植入式医疗器械市场参与者密度:了解其对业务动态的影响
植入式医疗器械市场正在快速增长,这得益于终端用户需求的不断增长,而这些需求的驱动因素包括消费者偏好的演变、技术进步以及对产品优势的认知度的提升。随着需求的增长,企业正在扩展产品线,不断创新以满足消费者需求,并抓住新兴趋势,从而进一步推动市场增长。
- 获取 植入式医疗器械市场 主要参与者概述
竞争格局和主要公司:
雅培实验室、波士顿科学公司、登士柏西诺德公司、强生公司、美敦力公司、士卓曼研究所、施乐辉公司、百多力公司、利瓦诺瓦公司和 MED-EL 医疗设备公司是植入式医疗器械市场的领军企业。这些公司专注于新技术、现有产品的改进和地域扩张,以满足全球日益增长的消费者需求,并扩大其专业产品组合的范围。
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST和SWOT分析
- 市场规模、价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
近期报告
相关报告
客户评价
购买理由
- 明智的决策
- 了解市场动态
- 竞争分析
- 客户洞察
- 市场预测
- 风险规避
- 战略规划
- 投资论证
- 识别新兴市场
- 优化营销策略
- 提升运营效率
- 顺应监管趋势






















 获取免费样品 - 植入式医疗器械市场
获取免费样品 - 植入式医疗器械市场