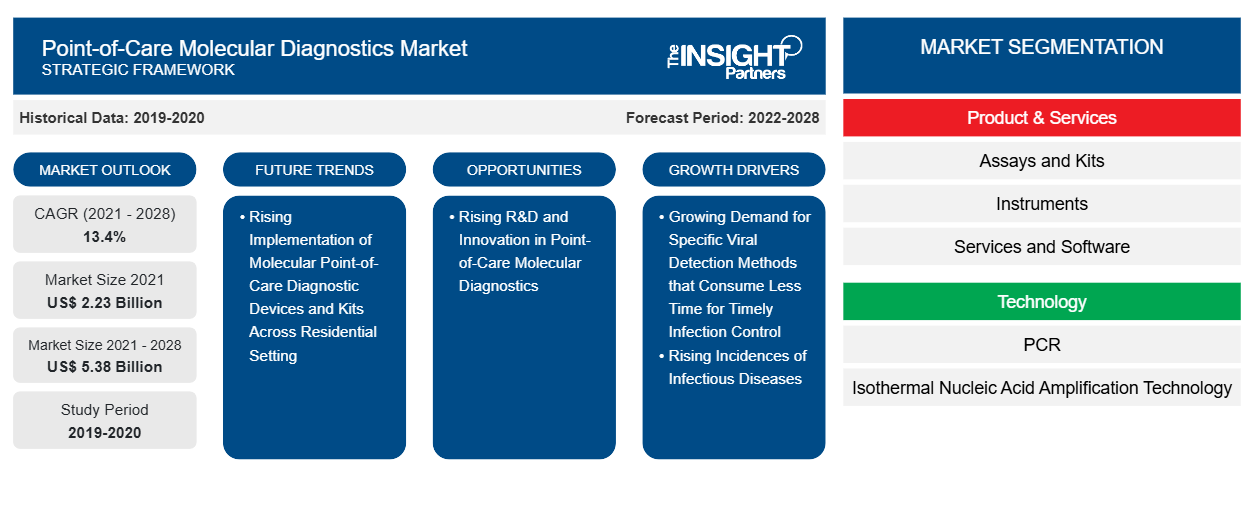
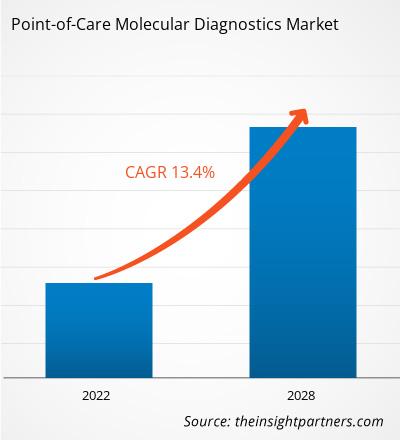
[研究报告] 即时诊断分子诊断市场预计将从 2021 年的 22.3094 亿美元增至 2028 年的 53.8118 亿美元;预计 2021 年至 2028 年的复合年增长率为 13.4%。
市场洞察和分析师观点:
即时诊断分子诊断包括便携式设备以及用于检测和诊断人体样本(例如咽拭子、血液、血清和粪便)中疾病的检测和试剂盒。由于分子诊断简单、方便、周转时间短以及改善患者预后的潜力,它正在从集中式实验室转向分散式即时诊断分子检测。由于这些优势,它可以应用于资源匮乏或偏远地区的诊断。传染病发病率的不断上升极大地推动了对有效诊断的需求。在预测期内,对控制和消除传染病、及时检测病原体、实现有效治疗和疾病控制的诊断工具的需求不断增加将推动市场发展。此外,现代技术正在使分子诊断领域发生显著的变革。全球即时诊断分子诊断市场规模预计将从 2021 年的 22.3094 亿美元增至 2028 年的 53.8118 亿美元。预计 2021 年至 2028 年期间市场复合年增长率为 13.4%。
增长动力和挑战:
传染病发病率上升
传染病的不断蔓延提高了检测水平和检测率。将传染病的分子诊断检测从实验室转移到即时诊断环境可能会彻底改变检测率和检测量。分子诊断是一种更灵敏的产品和服务,可以检测出浓度更低的传染性病原体,从而比以前更早地发现疾病。即时诊断分子诊断可以最大限度地缩短获得可操作结果所需的时间,并促进早期感染检测、适当的感染控制措施和治疗临床试验的参与。甲型/乙型流感、呼吸道合胞病毒 (RSV) 和医院内感染 (HAI) 的患病率不断上升,推动了即时诊断分子检测的需求。流感和呼吸道合胞病毒 (RSV) 即时诊断可以改善患者的治疗和感染控制。最近,即时诊断在检测 COVID-19 感染方面发挥了关键作用。对于 COVID-19 感染检测,基于 RT-PCR 的诊断测试耗时、昂贵,并且需要先进的设备和专业人员。诊断成本高昂且检测试剂盒稀缺,使得监测社区传播变得困难。因此,迫切需要快速、经济且有效的方法来检测人群中的 COVID-19 病毒感染。简单有效的即时分子诊断设备允许进行现场检测,这有助于预防感染和控制其传播。因此,对用于检测传染病的快速有效的即时分子试剂盒的需求不断增长,推动了市场增长。
定制此报告以满足您的需求
您可以免费定制任何报告,包括本报告的部分内容、国家级分析、Excel 数据包,以及为初创企业和大学提供优惠和折扣
- 获取此报告的关键市场趋势。这个免费样品将包括数据分析,从市场趋势到估计和预测。
市场限制
报销额度减少带来的定价压力
目前,医疗诊断市场正面临各种方法报销不足的问题,而这些方法对于患者的诊断至关重要。报销不足对市场产生了负面影响,主要经济体的大型市场增长持平。即时诊断分子诊断测试在全球各国都面临着类似的报销不足问题。报销制度通常不利于诊断测试。由于诊断测试报销不足,测试价格过低,最终降低了行业的盈利能力和市场规模。此外,低效的报销结构阻碍了更好诊断测试的发展。此外,报销削减可能会对临床实践产生负面影响,因为它既面临着不愿采用诊断方法的问题,也面临着未来缺乏改进测试的问题。此外,各国在具体治疗方面都有自己的报销结构和政策。例如,美国和德国的当局会补偿医院治疗一种疾病的全部费用。因此,如果患者住院时间超过预期,医院将承担费用。如果医院和医疗中心认为额外的测试会导致利润损失或每位患者的财务损失,这种情况可能会阻碍诊断工具的使用。因此,由于报销结构僵化,即时分子诊断设备的原始设备制造商 (OEM) 面临着众多官僚主义和定价挑战。因此,报销问题给即时分子诊断设备制造商带来了定价压力,这对即时分子诊断市场的整体增长产生了重大制约。
报告细分和范围:
“全球即时诊断分子诊断”根据产品和服务、技术、应用、最终用户和地理位置进行细分。根据产品和服务,全球即时诊断分子诊断市场细分为检测和试剂盒、仪器以及服务和软件。基于技术的即时诊断分子诊断市场细分为 PCR、等温核酸扩增技术 (INAAT) 和其他技术。基于应用的即时诊断分子诊断市场细分为传染病、肿瘤学、血液学、产前检测、内分泌学和其他应用。基于最终用户的即时诊断分子诊断市场细分为医院和诊所、诊断实验室、研究和学术机构等。
基于地理位置的健康市场商业化即时分子诊断分为北美(美国、加拿大和墨西哥)、欧洲(德国、法国、意大利、英国、俄罗斯和欧洲其他地区)、亚太地区(澳大利亚、中国、日本、印度、韩国和亚太地区其他地区)、中东和非洲(南非、沙特阿拉伯、阿联酋和中东和非洲其他地区)以及南美洲和中美洲(巴西、阿根廷和南美洲和中美洲其他地区)
节段分析:
根据技术,分子诊断即时诊断市场细分为 PCR、等温核酸扩增技术 (INAAT) 和其他技术。2021 年,PCR 细分市场可能会占据最大的市场份额,但 INAAT 细分市场预计将在预测期内以最快的速度增长。核酸的等温扩增可在恒定温度下快速有效地积累核酸序列。扩增技术是作为 PCR 的替代方案而开发的,用于生物传感靶标,例如 DNA、RNA、小分子、蛋白质、细胞和离子。等温扩增方法产生的扩增子可用于构建多功能核酸纳米材料,在生物医学、生物成像和生物传感方面具有多种应用。临床样本的复杂生化性质、临床样本中存在的低丰度核酸靶标以及现有的生物传感器技术表明,需要某种形式的核酸扩增才能从即时诊断中使用的小样本中获得临床相关的灵敏度。
根据产品和服务,即时分子诊断市场细分为检测和试剂盒、仪器以及服务和软件。2021 年,检测和试剂盒部分可能会占据最大的市场份额,预计在预测期内将以最快的速度增长。即时分子诊断检测和试剂盒专为医生办公室、医院重症监护室、门诊和社区卫生站设计。检测和试剂盒有助于早期诊断呼吸道感染和女性健康和性健康状况。检测试剂盒主要用于生命科学研究、环境监测和药物发现和开发。它们还用于各种应用,例如研究疾病途径、筛选潜在候选药物和评估生物制药生产过程。POC ELISA(酶联免疫吸附测定)试剂盒广泛用于检测和量化样本中的蛋白质和抗原。靶向特异性 ELISA 试剂盒用于简化免疫检测实验。
根据应用,即时诊断分子诊断市场细分为传染病、肿瘤学、血液学、产前检测、内分泌学和其他应用。2021 年,传染病领域可能会占据最大的市场份额,然而,预计未来几年肿瘤学将以最快的速度增长。全世界约有 5000 万人患有癫痫,这是全球最常见的神经系统疾病之一。北美年龄调整后的癫痫发病率为十万人中 16 人至十万人中 51 人年。年龄调整后的患病率为千分之 2.2 至千分之 41,具体取决于报告国家。部分性癫痫可能占新发癫痫的三分之二。社会经济地位较低的人群发病率较高。约 25% 至 30% 的新发癫痫被认为是由其他原因引起的或继发于其他原因。癫痫发病率在年轻人和老年人中最高,并且在 50 岁后稳步上升。老年人癫痫发作和癫痫的最常见原因是脑血管疾病。
苯二氮卓类药物(如地西泮、咪达唑仑或劳拉西泮)可作为持续性癫痫发作的一线药物。即使在完成备受期待的苯二氮卓类难治性癫痫持续状态随机试验(即已确定的癫痫持续状态治疗试验 (ESETT))后,最佳二线药物仍不清楚。二线药物包括磷苯妥英、丙戊酸、左乙拉西坦等。
临床分子诊断市场分析、区域分析:
根据地理位置,全球即时诊断分子诊断市场分为五个主要区域:北美、欧洲、亚太地区、南美和中美以及中东和非洲。在北美,美国是即时诊断分子诊断市场的最大市场。可穿戴医疗设备、芯片实验室技术的引入以及智能手机使用量的增加推动了美国市场对即时诊断分子诊断设备的需求。在未来几年内,随着用于识别艾滋病毒、结核病和疟疾等传染病的快速检测越来越多,该行业将发生转变,临床医生和患者可以在智能手机上查看结果并做出适当的治疗决策。诊断行业的最新进步正在推动美国即时诊断分子诊断市场的发展,旨在快速诊断,以便快速制定临床决策,协助制定治疗方案。
此外,美国是受 COVID-19 疫情影响最严重的国家之一。需要快速检测和发现病毒才能及时治疗。包括美国在内的全球都迫切需要能够实现分散、快速、灵敏且低成本的 COVID-19 感染诊断的即时诊断 (POC) 技术。因此,COVID-19 疫情为美国即时诊断分子诊断市场带来了丰厚的增长机会。此外,该国慢性病发病率的迅速上升是推动市场发展的主要因素。例如,根据美国癌症协会的数据,2020 年美国估计有 180 万例新癌症病例被诊断出来。乳腺癌、肺癌和支气管癌、前列腺癌、结肠和直肠癌、黑色素瘤和肝癌是常见的癌症。
此外,根据美国疾病控制与预防中心 2020 年发布的研究,大约有 3420 万美国人患有糖尿病。根据同一项研究,美国青少年患糖尿病的几率更高。此类疾病的流行将推动该国对即时分子诊断的需求。此外,近年来,创新和增强的医疗技术显着增加。由于这种扩展,开发了先进的医疗设备,并刺激了医疗保健业务的发现和突破。此外,美国拥有多家致力于尖端即时分子诊断的公司。这一因素有望进一步推动美国即时分子诊断市场的发展。
实时 PCR (qPCR) 的引入扩大了分子诊断在医学领域的应用范围。近年来,美国市场突破性的检测源于在 POC 环境中或患者附近进行的分子诊断检测。分子检测可以提高传统患者附近和快速诊断检测的特异性和敏感性,推动美国 POC 分子诊断市场达到新的高度。这种诊断检测在实验室环境中的使用越来越多,预计会增加其需求。对特定疾病的早期和精确诊断以提供适当治疗的需求增加将导致新技术的出现,从而推动美国 POC 分子诊断市场的发展。
行业发展和未来机遇:
以下列出了全球即时诊断分子诊断市场的主要参与者采取的各种举措:
- 2021 年 2 月,罗氏宣布向 FDA 申请 SARS-CoV-2 快速抗原检测的紧急使用授权,使医疗专业人员能够在护理点快速做出决策。
- 2021 年 3 月,生物梅里埃宣布其专门从事分子综合征传染病检测的子公司 BioFire Diagnostics 已获得美国食品药品监督管理局 (FDA) 对 BIOFIRE® RP2.1 面板的 De Novo 授权。
- 2020 年 11 月,Enzo Biochem 公布了一项分析结果,该结果显示,在公司专有的 GENFLEX™ 分子诊断平台上进行的测试能够成功检测出目前已知的 COVID-19 变体的存在。虽然公司的 PCR 测试无法区分不同的变体,但可以进一步分析阳性样本以识别变体。目前市场上的快速抗原测试不具备这种能力。
- 2021 年 5 月,Biocartis Group NV 宣布与全球科学主导的生物制药公司阿斯利康 (LSE/STO/Nasdaq: AZN) 签署了一项新的协议,旨在在 Biocartis 的欧洲和全球分销商市场选定的医院地点提供快速且易于使用的 Idylla™ EGFR 检测产品,以支持识别具有 EGFR 突变的患者。
即时分子诊断
即时诊断分子诊断市场区域洞察
Insight Partners 的分析师已详尽解释了预测期内影响即时诊断分子诊断市场的区域趋势和因素。本节还讨论了北美、欧洲、亚太地区、中东和非洲以及南美和中美洲的即时诊断分子诊断市场细分和地理位置。
- 获取即时诊断分子诊断市场的区域特定数据
即时诊断分子诊断市场报告范围
| 报告属性 | 细节 |
|---|---|
| 2021 年市场规模 | 22.3亿美元 |
| 2028 年市场规模 | 53.8亿美元 |
| 全球复合年增长率(2021 - 2028) | 13.4% |
| 史料 | 2019-2020 |
| 预测期 | 2022-2028 |
| 涵盖的领域 | 按产品和服务
|
| 覆盖地区和国家 | 北美
|
| 市场领导者和主要公司简介 |
|
即时诊断分子诊断市场参与者密度:了解其对业务动态的影响
即时诊断分子诊断市场正在快速增长,这得益于终端用户需求的不断增长,而这些需求又源于消费者偏好的不断变化、技术进步以及对产品优势的认识不断提高等因素。随着需求的增加,企业正在扩大其产品范围,进行创新以满足消费者需求,并利用新兴趋势,从而进一步推动市场增长。
市场参与者密度是指在特定市场或行业内运营的企业或公司的分布情况。它表明在给定市场空间中,相对于其规模或总市场价值,有多少竞争对手(市场参与者)存在。
在即时诊断分子诊断市场运营的主要公司有:
- 生物梅里埃公司
- F.霍夫曼-罗氏有限公司
- 丹纳赫公司
- Enzo Biochem 公司
- 雅培
免责声明:上面列出的公司没有按照任何特定顺序排列。
- 获取即时诊断分子诊断市场主要参与者概览
Covid-19的影响:
美国是北美地区新冠肺炎病例最多的国家。这对该地区的各个行业以及供应和分销链产生了负面影响。在疫情期间,生命科学公司将重点转向开发用于治疗危及生命疾病的新型药物。此外,对快速检测设备的需求也有所增加,这对北美即时分子诊断市场的增长起到了重要作用。此外,新冠肺炎的持续蔓延也刺激了即时分子诊断试剂盒的需求。这些试剂盒的采用正在推动新产品的开发和发布。2021 年 3 月,Eurofins 旗下 Clinical Enterprise, Inc. 获得了美国食品药品监督管理局 (FDA) 的紧急使用授权 (EUA),用于其 EmpowerDX COVID-19 家庭采集试剂盒的直接面向消费者 (DTC) 版本。同样,2020 年 7 月,Eurofins USA 的临床诊断部门宣布推出用于检测 SARS-CoV-2 的混合 PCR 检测,这将大大降低客户每次 PCR 检测的成本。
竞争格局和重点公司:
全球即时分子诊断市场的一些知名企业包括 bioMérieux SA、F. Hoffmann-La Roche Ltd.、Danaher Corporation、Enzo Biochem, Inc.、Abbott、binx health, Inc.、Meridian BioScience, Inc.、Biocartis、Quidel Corporation、Bio-Rad Laboratories, Inc. 等。这些公司专注于新产品的推出和地域扩张,以满足全球日益增长的消费者需求,并增加其专业产品组合的产品范围。它们在全球拥有广泛的业务,这使它们能够服务于大量客户,从而增加其市场份额。
- 历史分析(2 年)、基准年、预测(7 年)及复合年增长率
- PEST 和 SWOT 分析
- 市场规模价值/数量 - 全球、区域、国家
- 行业和竞争格局
- Excel 数据集
- Vessel Monitoring System Market
- Health Economics and Outcome Research (HEOR) Services Market
- Advanced Planning and Scheduling Software Market
- Space Situational Awareness (SSA) Market
- Neurovascular Devices Market
- Skin Tightening Market
- Lyophilization Services for Biopharmaceuticals Market
- 3D Audio Market
- Medical Collagen Market
- Ceramic Injection Molding Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends
Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America
Country Scope
This text is related
to country scope.
常见问题
In 2021, the assays and kits segment is estimated to account for the largest market share in the global point-of-care molecular diagnostics market. POC molecular diagnostic assays and kits are specially designed for point of care such as hospital critical care units, physician offices, outpatient clinics, and community health posts. POC molecular diagnostics assays and kits enable the early diagnosis of infectious diseases, cancer, and women’s health and sexual health conditions among others.
Asia Pacific is expected to be the fastest growing region in the point-of-care molecular diagnostics market. The growth of the point-of-care molecular diagnostics market in this region is primarily due to rising incidences of chronic diseases associated with geriatric population, expansion of key market players in this region, insufficient sophisticated central laboratory testing services, and potential cost effectiveness of POC molecular diagnosis.
Point-of-care molecular diagnostics include portable devices, and assays & kits used to detect and diagnose diseases in human samples, such as throat swab, blood, serum, and stool. Molecular diagnostics are shifting from centralized laboratories to decentralized point-of-care molecular testing, due to its simplicity, convenience, rapid turnaround time, and potential to improve patient outcomes.
Increasing demand for diagnostic tools to control and eliminate infectious diseases, timely detection of causative agent, allowing effective treatment and disease control will fuel the market during the forecast period.
Various companies have made organic growth strategies in the point-of-care molecular diagnostics market. Some of the activities undertaken by the company, which have promoted its growth are, launches, enhancements and expansion & relocation activities. Companies such as F. Hoffmann-La Roche Ltd, Danaher amongst others are some of the companies that have been implementing various organic strategies that have helped the growth of the company.
North America dominates the global point-of-care molecular diagnostics market. Ongoing efforts by major market participants to strengthen their market position is an important factor contributing to the major share. Furthermore, several clinical studies investigating the efficiency and accuracy of novel molecular tests are also expected to contribute towards the market growth over the forecast period. Growing demand for rapid diagnosis and development of newer molecular diagnostic tests for DNA analysis have poised the region as a major market space.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Point-of-Care Molecular Diagnostics Market
- bioMérieux SA
- F. Hoffmann-La Roche Ltd.
- Danaher Corporation
- Enzo Biochem, Inc.
- Abbott
- binx health, Inc.
- Meridian BioScience, Inc.
- Biocartis
- Quidel Corporation
- Bio-Rad Laboratories, Inc.
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published and advised several client across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organization are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:
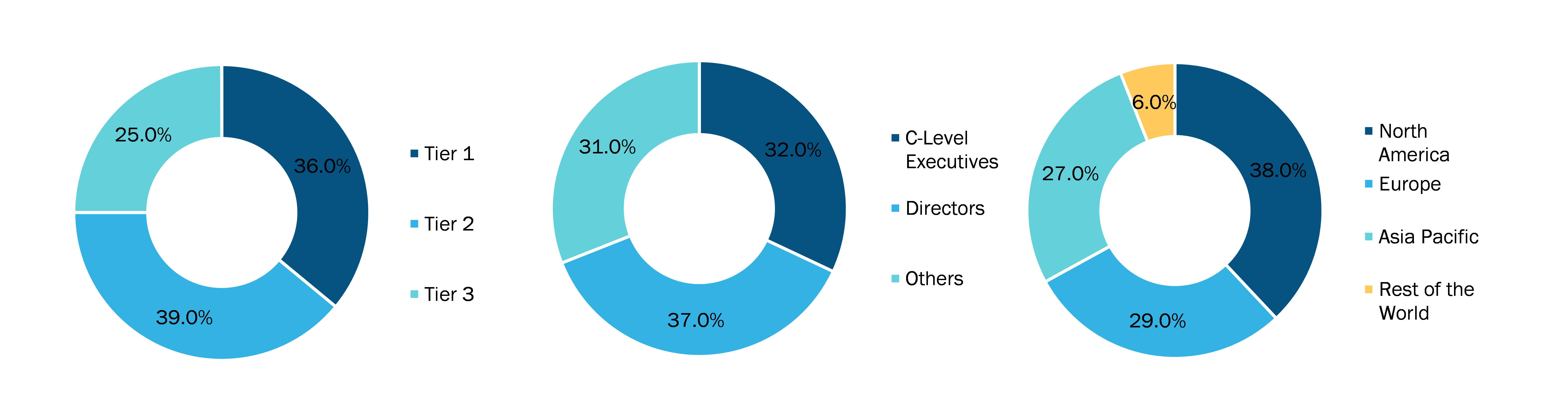
Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
 获取此报告的免费样本
获取此报告的免费样本