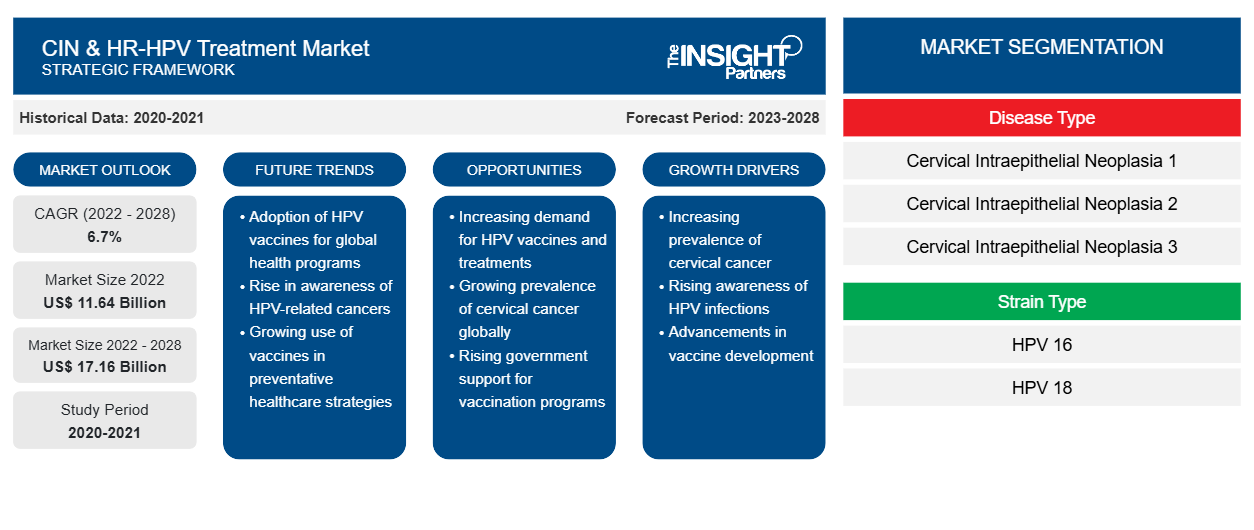
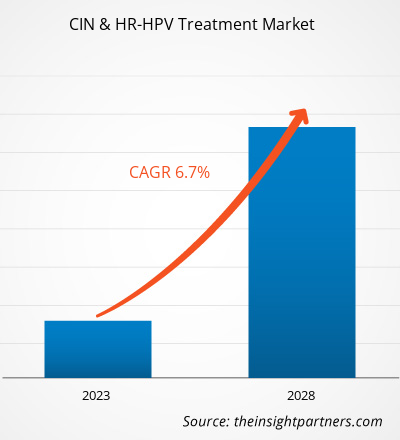
[Research Report] The CIN & HR-HPV treatment market size is expected to reach US$ 17,164.61 million by 2028 from US$ 11,635.17 million in 2022; the market is estimated to record a CAGR of 6.7% from 2023 to 2028.
The CIN & HR-HPV treatment market is segmented on the basis of disease type, strain type, offerings, product type, end user, and geography. The report offers insights and in-depth analysis of the market, emphasizing parameters such as drivers, trends, and opportunities in the market and competitive landscape analysis of leading market players across various regions. It also includes analyses of the impact of the COVID-19 pandemic across major regions.
Market Insights
Favorable Initiatives for Preventing Cervical Cancer Drives CIN & HR-HPV Treatment Market Growth
In August 2020, the World Health Organization (WHO) devised a global strategy for the elimination of cervical cancer. Per this strategy, all countries in the world must focus on reaching and maintaining an incidence rate of less than 4 per 100,000 women to eliminate cervical cancer. To attain such low incidence rates, countries should focus on cervical cancer screening, treatment, and vaccination. Medicare, a popular government insurance program, provides coverage for PAP tests, pelvic exams, and clinical breast examinations performed every two years for cervical cancer screening.
Unitaid, a global health agency that works to bring about innovative solutions to prevent, diagnose, and treat major diseases in low- and middle-income countries, has come up with a new initiative for cervical cancer prevention. Its efforts are focused on expanding access to critical tools and services to screen for the early signs of cervical cancer, followed by the treatment of positive cases. Unitaid is providing support for the Clinton Health Access Initiative (CHAI) and the Scale Up Cervical Cancer Elimination with Secondary Prevention Strategy (SUCCESS) Project, which are being carried out in partnership with Expertise France, Jhpiego, and the Union for International Cancer Control (UICC). Unitaid is collaborating with these partners and governments of 14 low- and middle-income countries to develop an affordable and highly effective package of tools, which can help the World Health Organization achieve its cervical cancer elimination targets. The Unitaid has invested ~US$ 70 million in innovative tools to screen women living in low-resource environments for precancer conditions and treat them. In 14 countries, Unitaid is striving to overcome access barriers and laying the groundwork for national cervical cancer elimination efforts, demonstrating effective models of prevention across low- and middle-income countries.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
CIN & HR-HPV Treatment Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
CIN & HR-HPV Treatment Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
In India, screening methods such as cytology, co-testing (HPV + cytology), primary HPV testing, and visual inspection with acetic acid are employed in various settings, according to resource availability and compliance standards. The Federation of Obstetricians and Gynaecologists of India (FOGSI) has published good clinical practice recommendations (GCPR) for the management of screen-positive women in different resource settings. RMNCH + A (which stands for Reproductive Maternal Neonatal Childhood Health + Adolescent Health + Adolescent) is the strategy of the National Health Mission of India (NHM) that encourages small family norms. This strategy involves delivering contraceptives at home by Accredited Social Health Activists (ASHA), delaying marriage age, promoting menstrual hygiene and sexual hygiene awareness, and prompting the treatment of reproductive tract illnesses in "Suraksha Clinics."
Such initiatives by various healthcare organizations and governments for preventing cervical cancer boost the growth of the CIN & HR-HPV treatment market.
Disease Type-Based Insights
Based on disease type, the CIN & HR-HPV treatment market is segmented into cervical intraepithelial neoplasia 1 (CIN 1), cervical intraepithelial neoplasia 2 (CIN 2), and cervical intraepithelial neoplasia 3 (CIN 3). In 2022, the cervical intraepithelial neoplasia 3 (CIN 3) segment held the largest market share, and it is anticipated to register the highest CAGR during the forecast period.
Strain Type-Based Insights
Based on strain type, the CIN & HR-HPV treatment market is segmented into HPV 16, HPV 18, and others. The HPV 16 segment held the largest share of the market in 2022. Th HPV 18 segment is expected to record the highest CAGR during the forecast period.
Offering-Based Insights
Based on offering, the CIN & HR-HPV treatment market is categorized into diagnostic method and treatment. In 2022, the treatment segment held a larger share of the market. However, diagnostic method segment is expected to register a higher CAGR during the forecast period. Further, the diagnostic method segment is further categorized into HPV testing, Pap smear test, colposcopy, and biopsy. The HPV testing segment held the largest share on the market for diagnostic method in 2022. In addition, the treatment segment is bifurcated into excisional surgery and ablative techniques. In 2022, the ablative techniques segment held a larger share of the market and is anticipated to register a higher CAGR in the market during the forecast period.
Product Type-Based Insights
Based on product type, the CIN & HR-HPV treatment market is segmented into kits & reagents, instruments, and services. The services segment held the largest share of the market in 2022, and it is expected to record the highest CAGR during the forecast period.
End User-Based Insights
Based on end user, the CIN & HR-HPV treatment market is segmented into hospitals & clinics, diagnostic laboratories, specialized clinical laboratories, and others. The hospital & clinics segment accounted for the largest market share in 2022. The diagnostic laboratories segment is expected to record the highest CAGR during the forecast period.
The CIN & HR-HPV treatment market players adopt organic strategies such as product launch and expansion to expand their geographic footprint and product portfolios. Additionally, these growth strategies help companies strengthen their clientele and enlarge their product portfolios.
- In October 2022, Fujirebio Europe and Self-screen BV formed a commercial collaboration to distribute the PreCursor-M+ methylation-specific molecular in-vitro diagnosis (IVD) assay (a trademark of Self-screen BV). The test is intended for qualitatively detecting the elevated methylation levels of cervical cancer biomarkers. It may be used as a triage follow-up test on women with HPV-positive results and ASCUS/LSIL cytology. The PreCursor-M+ assay completes Fujirebio's HPV-specific molecular test portfolio.
- In April 2021, Roche received US FDA approval for the fully automated, high throughput cobas 6800/8800 Systems for their use in HPV testing. The cobas HPV test identifies women at risk of cervical cancer by detecting the presence of hrHPV DNA in cervical samples. cobas HPV is indicated for routine cervical cancer screening per professional medical guidelines, including triage of ASC-US cytology and primary HPV screening of women to assess the risk for cervical precancer and cancer.
CIN and HR-HPV Treatment CIN & HR-HPV Treatment Market Regional Insights
CIN & HR-HPV Treatment Market Regional Insights
The regional trends and factors influencing the CIN & HR-HPV Treatment Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses CIN & HR-HPV Treatment Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
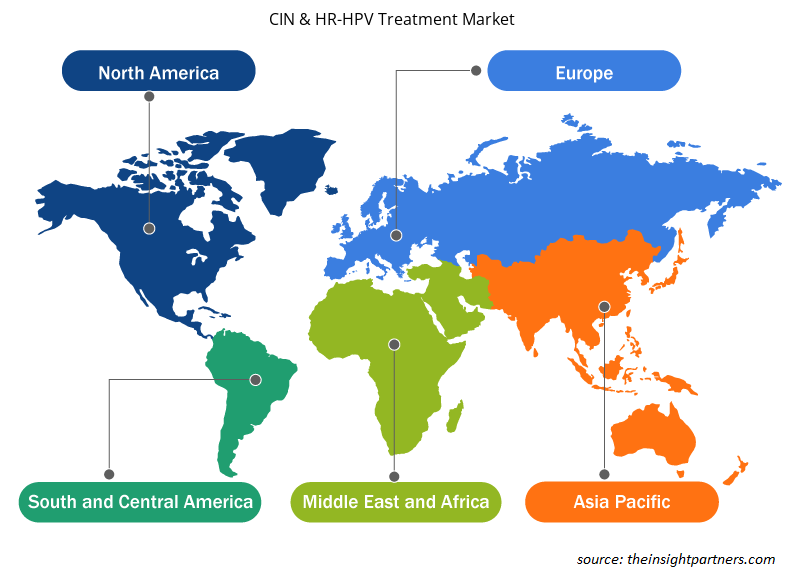
- Get the Regional Specific Data for CIN & HR-HPV Treatment Market
CIN & HR-HPV Treatment Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 11.64 Billion |
| Market Size by 2028 | US$ 17.16 Billion |
| Global CAGR (2022 - 2028) | 6.7% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Disease Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
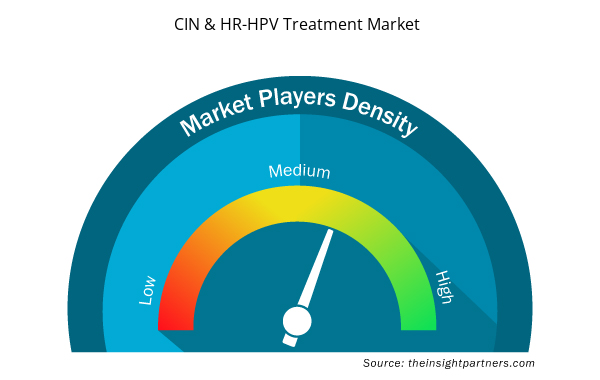
CIN & HR-HPV Treatment Market Players Density: Understanding Its Impact on Business Dynamics
The CIN & HR-HPV Treatment Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the CIN & HR-HPV Treatment Market are:
- Fujirebio Europe NV
- Qiagen NV
- Zilico Ltd
- Abbott Laboratories
- Cepheid
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the CIN & HR-HPV Treatment Market top key players overview
Company Profiles
- Fujirebio Europe NV
- Qiagen NV
- Zilico Ltd
- Abbott Laboratories
- Cepheid
- F. Hoffmann-La Roche Ltd
- INOVIO Pharmaceuticals Inc
- Bioneer Corp
- Antiva Biosciences Inc
- Thermo Fisher Scientific Inc.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Disease Type, Strain Type, Offering, Product Type, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
Key factors that are driving the growth of this market are Increase in prevalence of human papillomavirus infections and favorable Initiatives for preventing cervical cancer.
High-risk HPV is associated with cervical intraepithelial neoplasia (CIN). Persistent infection with high-risk human papillomavirus (HPV) results in invasive cervical cancer. Also, HR-HPV subtypes particularly HPV 16 and HPV 18 causes cervical cancer. The main aim of NHS Cervical Screening Program (NHS CSP) is to reduce the incidence and mortality from cervical cancer through a systematic, quality-assured population-based screening program for people aged 24 to 64.
Cervical Intraepithelial Neoplasia 3 segment held the largest share of the market in the global CIN and HR-HPV Treatment Market and held the largest market share of 47.48% in 2022.
The CAGR value of the CIN and HR-HPV Treatment Market during the forecasted period of 2022-2028 is 6.7%.
The hospitals & clinics segment dominated the global CIN and HR-HPV Treatment Market and held the largest market share in 2021.
Global CIN and HR-HPV Treatment Market is segmented by region into North America, Europe, Asia Pacific, Middle East & Africa and South & Central America. North America held the largest market share of the CIN and HR-HPV Treatment Market in 2021. With several North American market players focusing on research and development activities, the regional market for CIN and HR-HPV Treatment Market is likely to propel in North America region during the forecast period.
Abbott and Cepheid are the top two companies that hold huge market shares in the CIN & HR-HPV Treatment Market.
The CIN and HR-HPV Treatment Market majorly consists of the players such Fujirebio Europe NV, Qiagen NV, Zilico Ltd, Abbott Laboratories, Cepheid, F. Hoffmann-La Roche Ltd, INOVIO Pharmaceuticals Inc, Bioneer Corp, Antiva Biosciences Inc, and Thermo Fisher Scientific Inc.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - CIN & HR-HPV Treatment Market
- Fujirebio Europe NV
- Qiagen NV
- Zilico Ltd
- Abbott Laboratories
- Cepheid
- F. Hoffmann-La Roche Ltd
- INOVIO Pharmaceuticals Inc
- Bioneer Corp
- Antiva Biosciences Inc
- Thermo Fisher Scientific Inc.
 Get Free Sample For
Get Free Sample For