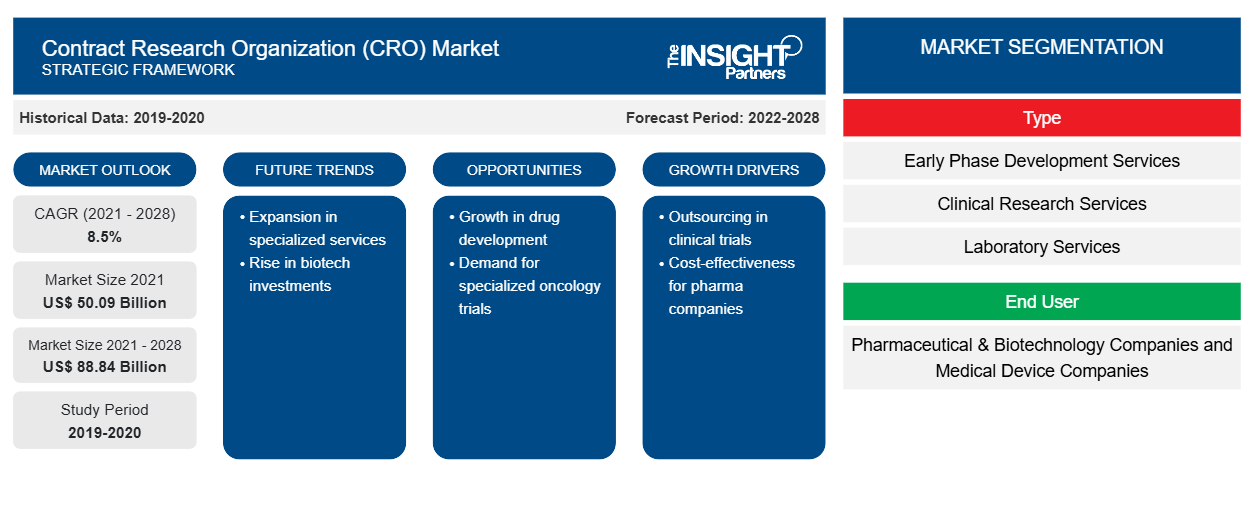
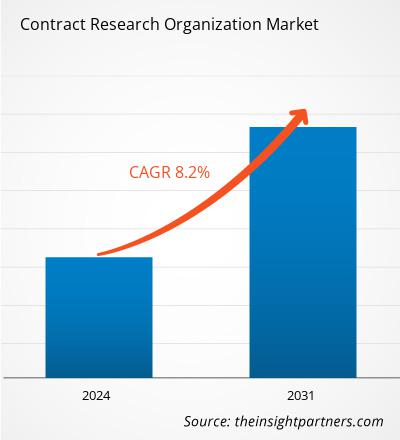
The contract research organization market size is projected to reach US$ 113.79 billion by 2031 from US$ 65.39 billion in 2024. The market is estimated to register a CAGR of 8.2% during 2024–2031. The sustainability initiatives are likely to bring new trends to the market in the coming years.
Contract Research Organization Market Analysis
The global contract research organization (cro) market is experiencing significant growth momentum as pharmaceutical and biotechnology companies increasingly leverage external partners for research and development assistance. This not only increases efficiency but also offers access to technical expertise that is in short supply and increasing specialized expertise, which aid in managing the changing market landscape.
Contract Research Organization Market Overview
According to the data provided by the National Library of Medicine (NLM), ~52,000 new studies were registered with NLM (ClinicalTrials.gov) in 2020, which increased to ~58,000 in 2023. In January 2023, the NLM reported 38,837 active clinical trials in the US and 105,172 active trials worldwide. According to the European Medicine Agency, in the European Union (EU), ~4,000 clinical trials are authorized annually, of which ~60% of clinical trials are associated with the pharmaceutical industry. An increasing number of clinical trials for developing different effective treatments due to the rising prevalence of chronic diseases globally is fueling the growth of the global CRO market. With bigger sample sizes, diversified patient demographics, and multiple study sites, clinical trials are becoming more complex.
Managing the various aspects of trials, from oncology research to vaccine development, can overwhelm sponsors. CROs enhance trial efficiency and productivity while handling day-to-day research activities that may be impractical or costly for drug developers to conduct in-house, including trial design, bioanalytical testing, and regulatory consultation. The growing investments and collaborations by market players to enhance clinical trials are fueling the growth of the market. In February 2025, the Society for Clinical Research Sites (SCRS) and Fortrea, a leading CRO, announced that Fortrea will sponsor the SCRS Collaborate Forward working group to enhance collaboration in clinical research. This group, made up of 16 global impact partner organizations, aims to reduce administrative burdens in clinical research by developing best practices. Their focus is on enhancing transparency and collaboration to improve site sustainability and trial efficiency, ultimately benefiting patients.
Therefore, the growing number of clinical trials is expected to boost the demand for CRO services to carry out complex studies at lower costs during the forecast period.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Contract Research Organization Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Contract Research Organization Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Contract Research Organization Market Drivers and Opportunities
Increased Outsourcing of R&D Bolsters Market Growth
The Contract Research Organization (CRO) industry is growing strongly across the globe, owing to rising outsourcing of research and development (R&D) operations in pharmaceutical, biotechnology, and medical device firms. With the healthcare sector becoming increasingly competitive and innovation-driven, firms are under tremendous pressure to speed up drug discovery. Contracting R&D to CROs enables companies to access their specialized expertise, sophisticated infrastructure, and global connectivity without significant investments. CROs offer full-service solutions, from preclinical research to clinical trials, regulatory affairs, and post-marketing surveillance, and thus help sponsors concentrate on their core competencies. In addition, the increasing complexity of clinical trials due to the commonality of chronic diseases, orphan diseases, and personalized medicine has raised the demand for niche CROs with specialized knowledge and technological capacities. Syngene has a dedicated research facility BBRC for Amgen, Baxter, and Bristol-Myers Squibb for specialist discovery, development, and manufacturing facilities in Bangalore.
Discovery CROs are forming long-term strategic partnerships with pharmaceutical and biotechnology companies, academic institutions, and other CROs. These collaborations can involve co-developing drugs, joint ventures, or preferred provider agreements, allowing both parties to leverage each other’s strengths. In November 2024, Novotech, a global full-service clinical CRO, formed a long-term partnership with Beijing Biostar Pharmaceuticals Co., Ltd. This collaboration was aimed to advance clinical research by utilizing Novotech’s expertise to support Biostar’s clinical development plans for novel therapeutics.
Such partnerships can significantly reduce the time required to bring a drug to market, accelerating the process by months compared to traditional outsourcing. It typically takes 10 to 15 years and over US$ 2.5 billion to develop a single drug, with most costs arising from the discovery and development phases in the US. By sharing expertise, resources, and technologies, these collaborations foster innovation, help mitigate risks, and distribute research costs.
Therefore, the market is well positioned for continued growth due to rising dependence on R&D outsourcing to achieve efficiency, save costs, and get life-saving drugs to patients more quickly.
Expansion of Biologics and Biosimilars to Create Growth Opportunities
The growth potential for the CRO market is largely supplemented with the upcoming biologics and biosimilars, mainly due to the increased competition from innovative therapies and the expired patents of blockbuster biologics. Biologics include product types such as monoclonal antibodies and recombinant proteins, and these will rule the landscape of biopharmaceuticals mainly owing to their strengths in curing chronic and life-threatening diseases. With patent expiration for most biologics, the market for biosimilars, copies of biologics that are very similar and price-effective, is already developing rapidly. This is where the end; CROs stand to gain lucratively in outsourcing research and development for the faster delivery of biosimilars.
The high cost and lengthy process of developing biologics attract outsourcing to CROs, with companies looking to cut costs and shorten time-to-market. CROs investing in advanced technologies, biologics, and biosimilar expertise should expect to profit most from this expanding pool. The world's push for financially affordable health solutions continues to fuel this growth, with the cost savings generated by biosimilars that are therapeutically equivalent to their reference biologics. Therefore, the continuous increase in biologics and biosimilars is exciting the market and stressing the importance of CROs in advancing biopharmaceutical development and helping to meet unmet medical needs.
Contract Research Organization Market Report Segmentation Analysis
Key segments that contributed to the derivation of the contract research organization market analysis are service type, management model, and geography.
- Based on service type, the contract research organization market is segmented into early phase development services, clinical research services, laboratory services, and post-approval services. The clinical research services segment held the largest share of the contract research organization market in 2024, and it is expected to register a significant CAGR during 2024–2031.
- By product type, the market is categorized into cell and gene therapy, biosimilars, antibody drug conjugates, and others. The biosimilars segment held the largest share of the contract research organization market in 2024.
- Based on type, the contract research organization market is bifurcated into in-house and outsource. The outsource segment held a larger share of the contract research organization market in 2024, and it is expected to register a significant CAGR during 2024–2031.
- By application, the market is categorized into oncology, neurology, cardiology, infectious diseases, metabolic disorders, nephrology, respiratory, dermatology, ophthalmology, hematology, and others. The oncology segment held the largest share of the contract research organization market in 2024.
- Based on end user, the contract research organization market is segmented into pharmaceutical and biotech companies, medical device companies, academic and research institutes, and others. The pharmaceutical and biotech companies segment held the largest share of the contract research organization market in 2024, and it is expected to register a significant CAGR during 2024–2031.
Contract Research Organization Market Share Analysis by Geography
The geographic scope of the contract research organization market report is mainly divided into five major regions: North America, Europe, Asia Pacific, the Middle East and Africa, and South and Central America. The growth in North America is characterized by growing demand for contract research organizations by the healthcare market players, growing support from the government to provide cost-effective healthcare service, increasing strategic developments by the contract research organization players to enhance services, and a growing healthcare industry that demands frameworks and guidelines based on the real-world data for their business models. The US is the largest market for contract research organizations. It is estimated to continue its dominancy owing to the presence of leading contract research organizations. This well-established healthcare industry provides a broader scope for contract research organizations and a growing shift toward outsourcing R&D and clinical trial services.
Contract research organizations are crucial to the biotechnology, pharmaceutical, and medical device industries owing to their offering of outsourced research services that help accelerate the typically prolonged and complicated drug development process. Due to the need for quicker drug development and technical developments, the landscape for contract research organizations in the US is evolving quickly. The regulatory environment heavily impacts the operation of contract research organizations. Initiatives such as real-time assessment, which permits simultaneous data assessment during clinical trials, have been established by the US FDA. Contract research organizations manage and evaluate data in real time related to this change.
There are a growing number of clinical trials with increasing investments in research and development in the country. According to an article published in the United States National Library of Medicine in May 2023, 437,533 clinical trials were registered in 221 countries in 2023, an increase from 399,499 in 2022, among which 140,492 (31%) were registered in the US. Contract research organizations help lower the risks of trial delays and regulatory obstacles by assisting pharmaceutical businesses in navigating this environment.
Contract Research Organization Market Regional Insights
The regional trends and factors influencing the Contract Research Organization Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Contract Research Organization Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Contract Research Organization Market
Contract Research Organization Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 65.39 Billion |
| Market Size by 2031 | US$ 113.79 Billion |
| Global CAGR (2024 - 2031) | 8.2% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Service Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Contract Research Organization Market Players Density: Understanding Its Impact on Business Dynamics
The Contract Research Organization Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Contract Research Organization Market are:
- Parexel International Corp
- Thermo Fisher Scientific
- Precision Medicine Group, LLC
- ProPharma Group
- Medpace Holdings Inc
- 04 Research Ltd
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Contract Research Organization Market top key players overview
Contract Research Organization Market News and Recent Developments
The Contract Research Organization Market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. A few of the key developments in the market are listed below:
- Thermo Fisher Scientific Launched International CorEvitas Clinical Registry in Adolescent Alopecia Areata. The registry was made active in both Europe and the US. The first patient was enrolled in Europe. This marked the 12th independent registry from CorEvitas, which was part of the PPD clinical research division of Thermo Fisher Scientific. It complemented an existing CorEvitas registry that focused on adult AA, launched in 2023. (Source: Thermo Fisher Scientific, Company Website, February 2025)
- Julius Clinical Research announced a majority recapitalization by Ampersand Capital Partners (Ampersand). The investment and partnership with Ampersand enables the Company to expand its scientific and therapeutic expertise as well as build its geographic footprint in Europe and North America to accelerate the growth of its CRO services. (Source: Julius Clinical Research, Company Website, November 2023).
Contract Research Organization Market Report Coverage and Deliverables
The "Contract Research Organization Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Contract research organization market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Contract research organization market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Contract Research Organization Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the contract research organization market
- Detailed company profiles
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Biopharmaceutical Contract Manufacturing Market
- Fishing Equipment Market
- Sweet Potato Market
- Compounding Pharmacies Market
- Authentication and Brand Protection Market
- Emergency Department Information System (EDIS) Market
- Hair Wig Market
- Aerospace Forging Market
- Quantitative Structure-Activity Relationship (QSAR) Market
- Artificial Intelligence in Healthcare Diagnosis Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Type and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, RoAPAC, RoMEA, RoSCAM, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
North America dominated the market in 2024.
Rising demand for clinical trials and increased outsourcing of research and development are among the significant factors fueling the market growth.
Sustainability initiatives are expected to emerge as a prime trend in the market in the coming years.
Parexel International Corp; Thermo Fisher Scientific; Precision Medicine Group, LLC; ProPharma Group; Medpace Holdings Inc; 04 Research Ltd; Julius Clinical, Siron Clinical; Clinmark sp. z.o.o; Pharmaxi LLC; Aurigon GmBH; Smerud Medical Research Group; Syneos Health Inc; IQVIA Holdings Inc. are among the key players in the market.
The market value is expected to reach US$ 113.79 million by 2031.
The market is expected to register a CAGR of 8.2% during 2024–2031.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Contract Research Organization Market
- Parexel International Corp
- Thermo Fisher Scientific
- Precision Medicine Group, LLC
- ProPharma Group
- Medpace Holdings Inc
- 04 Research Ltd
- Julius Clinical
- Siron Clinical
- Clinmark sp. z.o.o
- Pharmaxi LLC
- Aurigon GmBH
- Smerud Medical Research Group
- Syneos Health Inc
- IQVIA Holdings Inc
 Get Free Sample For
Get Free Sample For