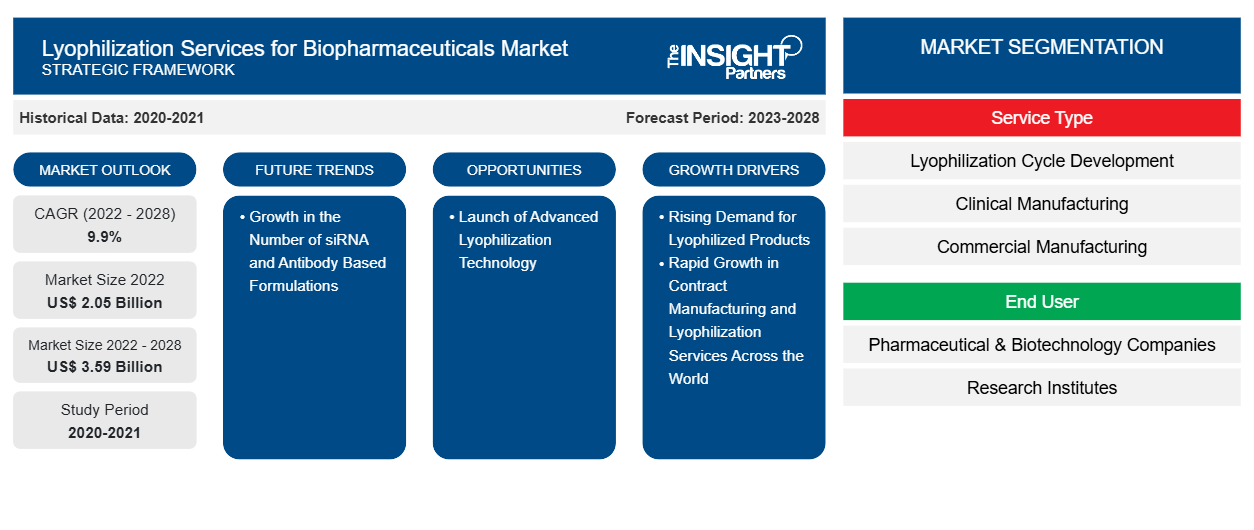
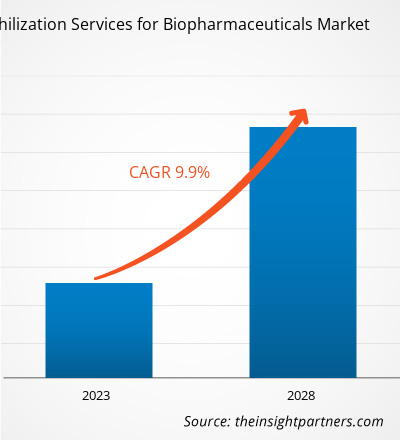
[Research Report] The lyophilization services for biopharmaceuticals market is expected to grow from US$ 2,051.41 million in 2022 to US$ 3,586.55 million by 2028; it is estimated to register a CAGR of 9.9% from 2023 to 2028.
The lyophilization services for biopharmaceuticals market growth is attributed to the rising demand for lyophilized products and rapidly growing contract manufacturing and lyophilization services across the world.
The lyophilization services for biopharmaceuticals market is segmented on the basis of service type, end user, and region. The report offers insights and in-depth analysis of the market, emphasizing parameters such as lyophilization services for biopharmaceuticals market trends, technological advancements, market dynamics, and the competitive landscape analysis of leading market players.
Lyophilization Services for Biopharmaceuticals Market –Market Insights
Rapid Increase in Contract Manufacturing and Lyophilization Services Drives Lyophilization Services for Biopharmaceuticals Market Growth
The biopharmaceuticals market continues to expand across the world, which can be the primary growth driver for the pharmaceutical industry. Biopharmaceutical parenteral manufacturing has expanded recently as more therapeutic biologics have been approved. Biotechnology companies outsource from contract manufacturing organizations (CMOs) to meet their fill-and-finish needs and reduce the risk of microbial contamination. Biopharma companies are dependent on contract manufacturing organizations (CMOs) to provide capacity and capabilities as needed; in some cases, CMOs provide a great deal of a company’s production.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Lyophilization Services for Biopharmaceuticals Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Lyophilization Services for Biopharmaceuticals Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Setting up in-house lyophilization capabilities and operations requires specialized equipment and expertise, which is an expensive and time-consuming. However, outsourcing is cheaper and increases the efficiency of manufacturing processes. Additionally, it allows biotechnology companies to redirect resources to other areas. Thus, drug developers and biopharmaceutical companies outsource such operations to CMOs to save overall production and yield cost. A few years ago, the CMO industry was a niche service market, offering additional manufacturing capacity or specific services to biotechnology companies. Now, many biotechnology companies outsource various services, from early-stage drug development to commercial-scale manufacturing. As the biotechnology industry is shifting from large-scale production to niche and targeted therapies (personalized medicine), the demand for flexible operational capabilities, production scales, and multiple-product operations is rising. Owing to all these factors, the inclination toward CMOs is growing. One such CMO that provides specialized facilities and dedicated lines for lyophilization operations is Jubilant HollisterStier Contract Manufacturing & Services. The CMO offers sterile fill/finish of Phase I through a commercial sterile injectable and a full suite of lyophilization services. To keep up with the growing demand for its services, Jubilant is installing a new 385-square-foot lyophilizer. Thus, the rising capability and availability of biopharma CMOs fuel the lyophilization services for biopharmaceuticals market growth.
Lyophilization Services for Biopharmaceuticals Market – By Service Type-Based Insights
Based on service type, the global lyophilization services for biopharmaceuticals market is segmented into commercial manufacturing, lyophilization cycle development, clinical manufacturing, and freeze drying analytical services. The commercial manufacturing segment held the largest market share in 2022. The lyophilization cycle development segment is expected to register the highest CAGR during the forecast period.
Lyophilization Services for Biopharmaceuticals Market – By End User-Based Insights
Based on end user, the global lyophilization services for biopharmaceuticals market is segmented into pharmaceutical and biotechnology companies, research institutes, and others. The pharmaceutical and biotechnology companies segment held the largest market share in 2022. The research institutes segment is expected to register the highest CAGR during the forecast period.
Companies in the lyophilization services for biopharmaceuticals market adopt inorganic and organic strategies such as mergers and acquisitions. A few recent key market developments are listed below:
- In November 2022, LTI announced its engagement in the development, process engineering, and preparation of clinical material for the tuberculosis vaccine candidate ID93 / GLA-SE. The tuberculosis vaccine has successfully reached Phase 2 clinical testing. This study represents the first report on the successful lyophilization of a thermostable subunit vaccine candidate containing an emulsion-based adjuvant.
- In May 2022, Jubilant HollisterStier LLC entered into a cooperative agreement for US$149.6 million with the Army Contracting Command, in coordination with the Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense (JPEOCBRND) on behalf of the Biomedical Advanced Research and Development Authority (BARDA), within the US Department of Health and Human Services.
- In October 2021, PCI Pharma Services (PCI) announced that they have signed a definitive agreement to acquire Lyophilization Services of New England, Inc. (LSNE), a premier contract development and manufacturing organization (CDMO) headquartered in Bedford, New Hampshire, from global private equity firm Permira. The acquisition adds five FDA-approved facilities in the US (New Hampshire, Wisconsin) and Europe (Spain), with a sixth expecting approval over the coming months. In addition, three additional facilities are under development. These facilities will strengthen their global 30-site network.
- In July 2021, Albany Molecular Research, Inc. (AMRI) announced that it is changing its name to Curia, effective July 12, 2021. The new name reinforces the company’s strategic positioning as an end-to-end global CDMO, applying its scientific expertise and extensive capabilities from research and development (R&D) to commercial manufacturing to enable its pharmaceutical and biotechnology customers to advance important new products that improve lives.
Lyophilization Services for Biopharmaceuticals Market Regional Insights
Lyophilization Services for Biopharmaceuticals Market Regional Insights
The regional trends and factors influencing the Lyophilization Services for Biopharmaceuticals Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Lyophilization Services for Biopharmaceuticals Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Lyophilization Services for Biopharmaceuticals Market
Lyophilization Services for Biopharmaceuticals Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 2.05 Billion |
| Market Size by 2028 | US$ 3.59 Billion |
| Global CAGR (2022 - 2028) | 9.9% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Service Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Lyophilization Services for Biopharmaceuticals Market Players Density: Understanding Its Impact on Business Dynamics
The Lyophilization Services for Biopharmaceuticals Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Lyophilization Services for Biopharmaceuticals Market are:
- ATTWILL Medical Solutions
- Axcellerate Pharma LLC
- Labyrinth Biopharma LLC
- Berkshire Sterile Manufacturing
- PCI Pharma Services
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Lyophilization Services for Biopharmaceuticals Market top key players overview
Company Profiles - Lyophilization Services for Biopharmaceuticals Market
- ATTWILL Medical Solutions
- Axcellerate Pharma LLC
- Labyrinth Biopharma LLC
- Berkshire Sterile Manufacturing
- PCI Pharma Services
- Curia Global Inc
- Emergent BioSolutions Inc
- Jubilant HollisterStier LLC
- Biofortuna
- Lyophilization Technology Inc.
- SYNERLAB GROUP
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Service Type, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, UAE, UK, US
Frequently Asked Questions
Based on service type, the global lyophilization services for biopharmaceuticals market is divided into commercial manufacturing, lyophilization cycle development, clinical manufacturing, and freeze-drying analytical services. The commercial manufacturing segment held the largest share of the market in 2022. The lyophilization cycle development segment is expected to grow at the highest CAGR during the forecast period.
Based on end user, the global lyophilization services for biopharmaceuticals market is divided into pharmaceutical and biotechnology companies, research institutes, and others. The pharmaceutical and biotechnology companies segment held the largest share of the market in 2022. The research institutes segment is expected to grow at the highest CAGR during the forecast period.
The growth of market is likely to grow due to factors such as rising demand for lyophilized products and rapid growth in contract manufacturing and lyophilization services across the world are expected to boost the growth of the global lyophilization service for biopharmaceuticals market. However, the market is likely to get impacted by the demerits associated with lyophilization process during the forecast period.
Companies operating in the market are ATTWILL Medical Solutions; Axcellerate Pharma LLC; Labyrinth Biopharma LLC; Berkshire Sterile Manufacturing; PCI Pharma Services; Curia Global Inc; Emergent BioSolutions Inc.; Jubilant HollisterStier LLC; Biofortuna; Lyophilization Technology Inc.; and SYNERLAB GROUP.
Lyophilization is a process of isolating a solid substance from a solution. It is also known as freeze-drying is a process. The process is carried by freezing the solution and evaporating under vacuum. It helps in optimizing lyophilization cycle times and improves stability for critically complexes of injectable during the parenteral product development.
Global lyophilization services for biopharmaceuticals market is segmented by region into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America. North America is likely to continue its dominance in the lyophilization services for biopharmaceuticals market during 2023–2028. The US holds the largest share of the market in North America and is expected to continue this trend during the forecast period.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Lyophilization Services for Biopharmaceuticals Market
- ATTWILL Medical Solutions
- Axcellerate Pharma LLC
- Labyrinth Biopharma LLC
- Berkshire Sterile Manufacturing
- PCI Pharma Services
- Curia Global Inc
- Emergent BioSolutions Inc
- Jubilant HollisterStier LLC
- Biofortuna
- Lyophilization Technology Inc.
- SYNERLAB GROUP
 Get Free Sample For
Get Free Sample For